Full Length Research Paper
ABSTRACT
Microbial remediation research has become front and centre gaining momentum for numerous environmental biotechnological applications. Agronomical bioproducts have been widely used for many years and their availability, low costs and safety makes them potentially suitable for alternative biotechnological applications, specifically environmental remediation. In this study, the commercially available biofertilizer, Pseudomonas fluorescens (Rizofos), was examined for its rhizosphere competence, abiotic stress tolerance and heavy metal tolerance using relatively rapid and economically feasible standard culture-based laboratory methods to determine their potential use in the field of bioremediation. This plant growth promoting bacteria biofertilizer shows phase variation, metabolic versatility, and mobility, all of which are necessary for rhizosphere fitness and colonisation. In addition, both exceptional abiotic stress tolerance (pH, NaCl and temperature) and heavy metal (HM) tolerance was exhibited by this biofertilizer. To conclude, this study demonstrates that the prospect of using already available, well studied, safe and environmentally friendly agronomic bioproducts as alternative biotechnologies including bioremediation is realistic offering a more rapid solution to environmental problems extending beyond identifying, testing and formulating new liquid bioinoculants specifically for contaminated soils.
Key words: Agricultural, bioproducts, Pseudomonas fluorescens, bioremediation.
INTRODUCTION
The economic growth of South Africa is primarily fuelled by land use activities, mining and non-renewable resource exploitation. However, the environmental impacts associated to these activities are extensive both in scale and severity. Natural resource utilization driven economic growth in South Africa urgently needs to become uncoupled from environmental degradation for sustainable development (Soderholm, 2020). Biotechnology offers a solution to overcoming the challenges associated with sustainable development in South Africa. However, biotechnology development and its integration into policy and management must be conceptualized and emerge upon the foundation of practical applicability in a developing-country context. Innovation and technology transfer offer such an avenue to allow the introduction, advent, and expansion of environmental biotechnology into South Africa.
The use of biofertilizers for sustainable agriculture represents one of the most advanced biotechnological tools in modern times. Across the world, at an annual increase of ~10%, there is an expanding market for microbial inoculants (Berg, 2009). When compared to chemical pesticides and fertilisers, microbial inoculants offer several advantages: They are safer with more targeted activity and reduced environmental damage. They are also effective in smaller quantities with the ability to multiply, and have decomposition procedures that are quicker (Berg, 2009).
The legacy of environmental pollution has followed in the wake of worldwide industrialisation and extensive agricultural and anthropogenic practices (Shinwari et al., 2015). Toxic compounds, metals and effluents have heavily contaminated soils, water and air leaving an environment that is unsuitable for sustaining life. Conventional methods for the remediation of contaminated soils and mine sites are high energy consuming, costly and in large unsustainable (Jeyasingh and Philip, 2005). In this regard, microbial based technologies, known as bioremediation, to remediate or assist remediation practices have gained considerable attention over the last few decades due to their superior performance, low cost, and environmentally friendly nature (Iqbal and Edyvean, 2004; Wu et al., 2006). Bioremediation is defined as the implementation of biological systems, almost universal microorganisms, to clean up or restore contaminated sites (Kumar et al., 2018).
The Pseudomonas genus is one of the best-studied and most important bacterial taxa in soil (Raaijmakers et al., 2002). The reasons for this are that the Pseudomonas species are isolated easily from the natural environment, effortless to cultivate and easy to manipulate genetically (Whipps, 2001; Raaijmakers et al., 2002). The members of the genus can colonise a wide range of niches as they demonstrate wide metabolic diversity (Palleroni and Cornelis, 2008). Other important characteristics of the genus Pseudomonas include rapid growth, production of metabolites (siderophores and growth promoters) and their ability to adapt to environmental stress and compete with other microorganisms (Goldberg, 2000; Kraemer, 2004; Hider and Kong, 2010; Cornelis, 2010; David et al., 2018). These features combine to make this microbial inoculant a strongly desired agent in applications such as bioremediation and biocontrol (Kloepper et al., 2004; Weller, 2007). Pseudomonas fluorescens is one species of this diverse group of bacteria that is particularly well known for its role in biocontrol, biodegradation, and bioremediation (Roca and Olsson, 2001; Palleroni, 2010).
The environmental biotechnological applicability of certain microbial species is generally performed by isolating soil microbes from the contaminated site and testing whether they possess the capacity to accomplish the desired function. This technique is however, limited by being site-specific and if the microbe presents as being applicable for bioremediation further time, money and workforce are required to developing the bacteria into a formulated inoculant that is safe for both the environment and human handling. This process is in all regard paradigmatic in the developing world where environmental conciseness and funds are not as broadly or extensively prioritized. Considering this, the use of commercially available, safe and relatively cheap PGPB biofertilizers exhibiting multiple plant beneficial properties, abiotic stress and metal tolerance is an encouraging, environmentally friendly and cost competitive soil bioremediating tool. The prospect of using commercially available biofertilizers for bioremediation technology and establishing whether the administration of PGPR biofertilizers, largely used to improve agricultural yields, for solving environmental problems is a concept that has in large been overlooked (Figure 1).
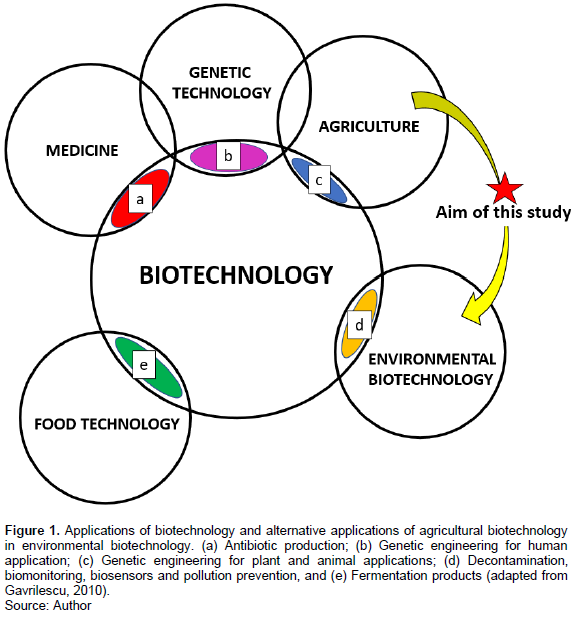
P. fluorescens containing biofertilizers produced specifically for their phosphate solubilising properties are a commercially available agricultural biotechnology (Goddard et al., 2001). Compared to fungi, the faster growth rate and ease for manipulation of bacteria make them convenient to culture. Thus, may serve potentially as more suitable effectors for the bioremediation of soil. Being commercially available and widely used, its performance for scale-up and field employment has already been confirmed and is a significant factor to why the commission of bioinoculants developed for biotechnological employment are commonly short-lived (Goddard et al., 2001). Numerous in-depth studies have exposed the bioremediation potentials of P. fluorescens (Juhasz and Naidu, 2000; Barathi and Vasudevan, 2001; Janek et al., 2010) and therefore this species was selected for this research. Thus, the microbial biofertilizer was investigated for its potential to be used beyond its current agricultural purpose in the realm of bioremediation and bioaugmentation-assisted rehabilitation of soils.
The aim of this work is to determine whether this biofertilizer shows morphological, behavioral, and metabolic phenotypes pertaining to rhizosphere colonization ability and survival. The objectives of this study were to use a culture-based strategy to examine colony morphology expressions, abiotic stress tolerance and heavy metal tolerance of P. fluorescens. PGPR P. fluorescens was cultured on a variety of variable culture media and colony growth times and the observed phenotypic expressions (colony morphology, alterations in colony traits, growth rate, fluorescent pigments and colouration) were examined and compared. Abiotic stress and heavy metal tolerance was also studied using standard culturing techniques and determined by adjusting the individual environmental parameters for each and inspecting the effects on growth. Identifying the impacts of some variables on the microbiological effects and phenotypic expressions of P. fluorescens provides inferences on their potential environmental adaptability, virulence and resistance following introduction into the soil environment (Sousa et al., 2013). As this microbe is already used in agriculture for its plant growth promoting abilities, these properties were not studied as the goal of this study was to determine if the use of this agricultural bioproduct in environmental biotechnological applications is a feasible recourse which mainly relying on its ability to survive, replicate, tolerate and perform the desired biotechnological appointment.
MATERIALS AND METHODS
Microorganism, maintenance and storage
The bacterial strain of P. fluorescens used in this study was donated as a commercially sold inoculant labelled Rizofos maize from Microbial Biological Fertilizers International (MBFi), Delmas, South Africa. Rizofos maize is a liquid inoculant containing the phosphorus solubilizing bacteria (Pseudomonas fluorescens) (1 × 109 cfu/mL in a sterile liquid broth culture). This strain is however also capable of producing siderophores under iron limited conditions a trait desired for this experiment. This biofertilizer is a special liquid formulation that contains not only the desired micro-organism and their nutrients, but also special cell protectants or substances that encourage longer shelf life and tolerance to stressful condition. The P. fluorescens was maintained at 4°C on nutrient agar (Merck, Darmstadt, Germany) plates. For long time storage, stock cultures were made from liquid cultures (1200 µl culture, 300 µl 80% glycerol) and frozen at -20°C.
Several growth-based assays were used to phenotypically characterise the P. fluorescens biofertilizer. These assays are important to increase understanding of the ecology of this significant rhizosphere related microorganisms and have major implications for its application as a bioinoculant for environmental restoration and remediation biotechnologies.
Media composition and preparation of experimental plates
P. fluorescens was cultured in four different media, Pseudomonas agar, Kings B agar, LB agar and nutrient agar to investigate the effects of the composition of different media and growth time on (i) morphological features (that is, colony growth, colour and edge), (ii) rate of bacterial growth and (iii) physiological features (that is, colour changes and fluorescens). To assess the impact of solid media composition on colony morphology, growth and fluorescens, P. fluorescens was streaked on different solid media (Table 1).
The media were prepared, autoclaved at 121°C for 20 min, allowed to cool and poured into Petri dishes under sterile conditions in triplicate. As the amount of solid medium in the Petri plates has been observed to impact colony morphogenesis (Sousa et al., 2013), the height of the solid media in each plate was standardised to 0.5 cm thick or ~15 mL of medium per 9 cm diameter plates with an area of 7226.4 mm2.
Detection of motility
Motility can be divided into (i) swimming and (ii) swarming forms (Robertson et al., 2013). A positive result for swimming motility is shown by a ring of colony expansion after 24 to 48 h of growth. Swarming motility is observed if the edge of isolated colonies demonstrates irregular extensions and/or projections after 24 to 48 h of growth. Colony morphogenesis and motility were monitored visually over the incubation period.
Detection of fluorescence
After 24, 48 and 72 h of incubation, the plates were examined for fluorescence under an ultraviolet light at 360 nm.
Abiotic stress tolerance
pH tolerance tests
To study the effect of temperature on growth of P. fluorescens, a loopful of 24-h old culture growth in nutrient broth were streaked onto nutrient agar plates with different pH values (4, 5, 6, 7, 8, 9 and 10). The final pH of the medium was adjusted using 1 mol/L HCl or 1 mol/L NaOH. The plates were incubated at 25°C for 48 h and visually inspected for the absence or presence of growth, which were then documented as intolerant (I) and tolerant (T), respectively.
Temperature tolerance tests
To study the effect of temperature on growth of P. fluorescens, a loopful of 24-h old culture growth in nutrient broth were streaked onto nutrient agar plates and incubated at different temperatures of 5, 10, 15, 20, 25, 30, 35 and 40°C for 48 to 96 h (Bruno et al., 2020). The plates were visually inspected for the absence or presence of growth which was then documented as intolerant (I) and tolerant (T), respectively.
Salinity tolerance tests
To study the effect of temperature on growth of P. fluorescens, a loopful of 24-h old culture growth in nutrient broth were streaked onto nutrient agar plates amended with different concentrations of NaCl (0.1, 1, 2, 3, 4, 5, 6 and 7%). The plates were incubated at 25°C for 48 h and visually inspected for the absence or presence of growth which was then documented as intolerant (I) and tolerant (T), respectively.
Heavy metal (HM) tolerance
The heavy metals used in the tolerance tests were iron, manganese, cobalt, zinc, copper, lead and chromium from FeSO4.6H2O, MnSO4.4H2O, CoCl2.6H2O, ZnCl2, CuO4S.5H2O, Pb(NO3)2 and CrO3, respectively. A 1 mol/L stock solution for each metal salt was made from which the appropriate volumes were taken to get the desired concentration of heavy metals in each of the 25 mL agar plates using C1V1 = C2V2 (Table 2).
The heavy metal (HM) tolerance of the PGPR P. fluorescens was tested using the agar dilution method (Lee et al., 2009). The P. fluorescens was grown in a nutrient broth for 24 h and then, using an inoculating loop, streaked onto nutrient agar plates amended individually with increasing concentrations (10, 50, 100, 200 and 300 ppm) of different heavy metals. Unamended nutrient agar plates were used as controls to examine tolerance. The plates were incubated at 25°C for 48 h and visually inspected for the absence or presence of growth which was then documented as intolerant (I) and tolerant (T), respectively.
RESULTS
Effect of medium composition on colony morphology, colour and growth
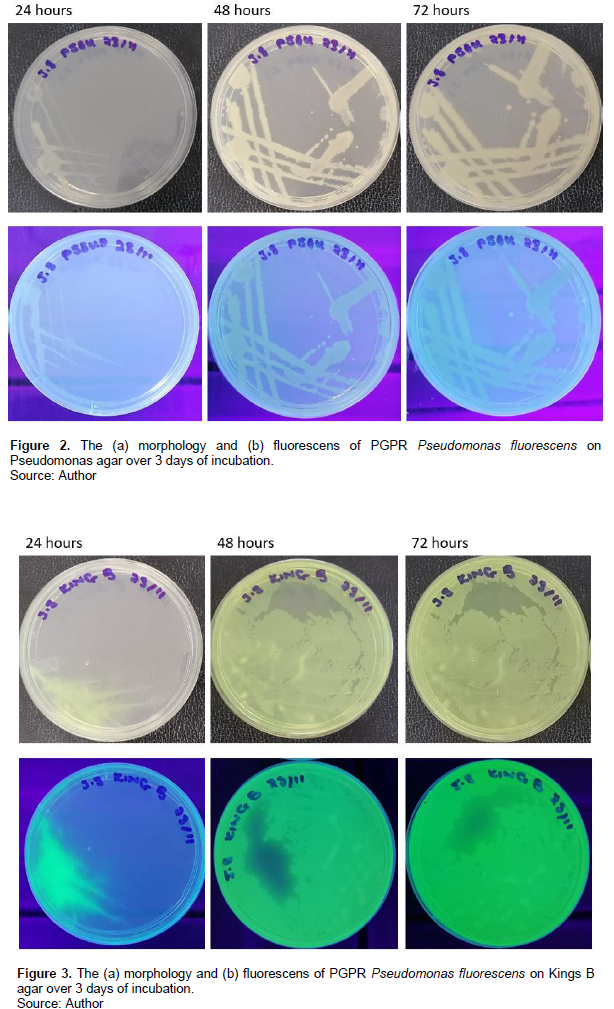
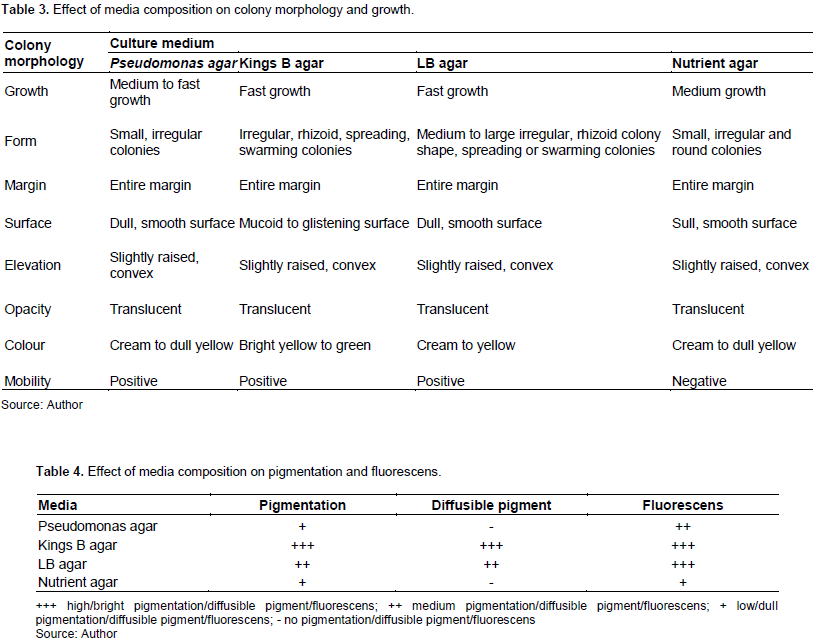
The PGPR P. fluorescens was plated onto different agar media to assess the effect of nutritional composition on colony morphology and biomass production. The time-lapse images of the plates at 24, 48 and 72 h are given for each medium in Figures 2 to 5.
Colony growth and expansion of the P. fluorescens strain over the course of 72 h showed variations in rate and extent amongst the four media types. Colony growth, degree of expansion and rate of expansion was greatest on the Kings B agar medium and slowest on the nutrient agar medium.
Motility was not observed on the nutrient agar medium. Swimming motility was observed on the Pseudomonas agar medium. The Kings B agar media was dominated by swarming motility growth. The LB agar medium showed both swimming and swarming motility and growth behaviours. Unlike the thick irregular tendrils observed on the Kings B agar, those on the LB agar plates where longer and slenderer. Colony growth and expansion on the Pseudomonas agar medium showed three stages beginning with an initial slow stage (24 h), followed by a fast stage (48 h) and lastly a slow stage (72 h) (Figure 2). The colony spread observed on this medium was swimming dependent with colony growth and expansion occurring as distinct, expanding rings. Colony growth and expansion on the Kings B agar plates showed an initial slow stage up until ~ 24 h of incubation after which rapid growth and expansion occurred to nearly cover the plate (Figure 3). Colony growth and expansion on the LB agar increased gradually and steadily over the 72-h incubation period (Figure 4). However, compared to that observed on the nutrient agar the growth and expansion rate was faster. Several irregular swarming extensions appeared at different sites on the swimming colony boarders at 48 h resulting in further expansion from the primary streak culture across the plate. Colony growth and expansion on the nutrient agar medium increased gradually and steadily over the 72-h incubation period as shown in Figure 5. The growth observed on the nutrient agar medium appears to be non-motile which also explains the lack of colony expansion across the agar surface.
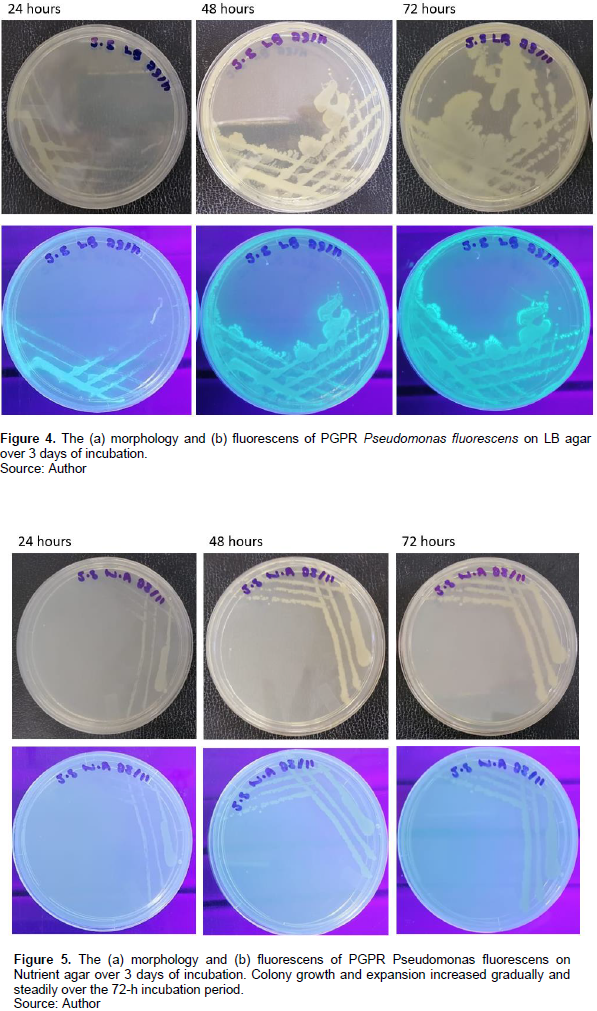
The results indicate that all the media used were suitable for the growth of P. fluorescens however the type and composition of the solid media used noticeably influence the colony morphogenesis of P. fluorescens as exhibited by distinct colony morphologies and growth patterns according to the culture media used (Table 3).
The motility, auto-aggregation, colour and pigment production were features that where visually verified to be the most strikingly affected (Table 4).
The results indicate that the PGPR P. fluorescens is a fast growing, nutritionally versatile, pigment producing, fluorescing bioinoculant that exhibits phase variation all of which strengthen its ability to survive, colonise and proliferate in the soil environment following introduction.
Abiotic stress tolerance and growth
The pH, temperature and salinity tolerance and growth limitations of the PGPR P. fluorescens are given in Table 5.
Growth was observed for a pH ranging between 5 (acidic) and 8 (alkaline), which indicates that it is not bound to a specific pH region and thus should not be sensitive to pH fluctuations above or below of neutral (pH 7) which are common in soil remediation methods. Extreme pH values of acidity and alkalinity would however impede the survival of this bioinoculant, regardless of liming and/or addition of alkaline amendments typically used during the rehabilitation of pH extreme sites. This biofertilizer exhibits temperature tolerance between 5 and 35 ± 2°C, which is sufficient for soil environments. Salinity tolerance was restricted to 4% NaCl, which allows the use of this biofertilizer to all but sodic soils. As sodic soils are not typically associated with metal sites or metalliferous mining areas this results only provides information for the few cases where the PGPR P. fluorescens should not be considered as a biological inoculant.
Heavy metal (HM) tolerance
The HM tolerance and growth limitations of the PGPR P. fluorescens are given in Table 6. The microbial inoculant demonstrated 100% tolerance to all concentrations for all the HM tested except chromium, with concentrations > 200 ppm inhibiting growth. This finding once again allows us to identify contaminated soils and/or mine sites where the use of PGPR P. fluorescens would be suitable. As different soils and mine lands possess unique geochemical properties and heavy metal associations understanding of HM and the degree of their concentration effects will allow determining the usefulness of a particular microbial agent as the appropriate inoculants.
DISCUSSION
In context of developing countries such as South Africa environment-based biotechnologies being economically and technically feasible for economic growth and environmental preservation are urgently demanded. Despite this necessity, the environmental biotechnology market for industrial sector application in South Africa is absent. Developing-country limitations to national environmental biotechnological capabilities must be acknowledged and the development of novel and leading environmentally sound technology undertaken in accordance.
For decades the introduction of desired bacteria and fungi into the soil has occurred for agricultural purposes (Chaudhary et al., 2020). These inoculations have been done to stimulate plant growth, supply nutrients, improve the structure of soil and control plant pathogen activity (Chaudhary et al., 2020). In more recent years, there have been other objectives for the introduction of microbial organisms into the soil (Rizvi et al., 2022). These include the bioaccumulation of inorganic compounds, microbial leaching and the bioremediation of pollutants in the soil (Nadeem et al., 2016).
Successful soil augmentation requires the selection of a microbial inoculant that is easily cultured; fast growing; occur and survive in a wide range of environmental conditions and can withstand high concentrations of contaminants (Mrozik and Piotrowska-Seget, 2010). The survival of strains introduced into the soil is one of the major difficulties associated with bioaugmentation procedures (Thompson et al., 2005). The effectiveness of bioaugmentation is influenced by both biotic and abiotic factors and the effect the have on the survival of the introduced microorganisms (Bento et al., 2005). Moreover, the efficiency of bioaugmentation is also determined by the soil type; nutrient content and aeration (Bento et al., 2005). Being an in-situ treatment, bioaugmentation, provides a safe and economic alternative to commonly used physicochemical strategies (Adams et al., 2015).
Insight into microorganisms displaying multifaceted beneficial traits such as plant growth promotion, rhizosphere competence, abiotic stress tolerance and/or heavy metal stress tolerance is of great importance in the realm of environmental biotechnology. Such microorganisms present novel agents in regulated and legally obligated ecological restoration efforts. The prospect of identifying and make use of already available and commercially sold bioproducts for environmental applications other than in agriculture is significant, especially in developing countries where research and funds restrict remediation practices. To the best of our knowledge this study is the first of its kind with regards to exploring the bioremediation potential of agronomic products sold in South Africa.
Phenotypic or phase variation is an important characteristic demonstrated in rhizosphere competent bacteria as it allows the achievement of population diversification within a species crucial in niche adaptation and enhance bacterial fitness when subjected to certain unfavourable conditions (Van den Broek et al., 2005). Colony morphotyping has been shown to be a definitive technique for assessing phenotypic variation which is an important measure colonization, survival and adaptive competence in the soil system (Sousa et al., 2013). The colony morphology method was applied in this study as means of rapidly predicting the competence of a selected strain and therefore its enduring performance following introduction into the field.
During colonisation of the different media, P. fluorescens underwent phenotypic variation which resulted in the emergence of colonies having different morphologies. The appearance of colony morphology variation of the P. fluorescens on and between each of the different media, representing structured environments, demonstrates the capability for both stress tolerance and niche specialisation. Additionally, the diffuse morphology observed for some colonies indicates that motility on the agar substrates may have occurred. This is not an unusual observation as P. fluorescens possess flagella required for swarming and swimming (Santoyo et al., 2021). Mobility has several advantages related to rhizosphere competence as it allows the microbe to colonise a greater area of the rhizosphere and populate potentially less microbially dense parts under competitive stress (Sánchez-Contreras et al., 2002). Colony colour and pigmentation variation was also observed for P. fluorescens on the different agar substrates. Under UV light the P. fluorescens demonstrated a blue pigmentation and fluorescence on the Pseudomonas agar, LB and nutrient agar and a vibrant green on Kings B. Pigmentation and fluorescence appears to be linked to the nutritional composition of the growth media. Pigment production is an important parameter as it is correlated to stress tolerance and survival under different environmental conditions (Ahmad et al., 2016).
Environmental stress is a major challenge for the sustainability of rehabilitation and therefore ecosystem recovery of mine sites, soils and rock wastes (Goswami and Deka, 2020; Khan et al., 2020). For a microbial inoculant to be considered effective in stress environments it is necessary that they display the ability to survive in adverse conditions. The results of this study suggest that the commercially available PGPR P. fluorescens demonstrates such a capacity for survival and persistence in unfavourable abiotic environments further supporting it suitability in applications of bioremediation.
The global repositioning to a greener economy and the subsequent biotechnological potentials of heavy metal tolerant microbes and their cellular products provided major momentum into their research. In general, microorganisms have many beneficial applications in the environment and are in large used for agricultural purposes such as promoting crop yields and/or increasing soil fertility (Nazli et al., 2020). However, biotechnological processes in large require that for microbial species to play a significant role, especially in matters regarding environmental and ecosystem recovery and/or heath, they need to demonstrate heavy metal tolerance (Nazli et al., 2020). Our study shows that PGPR P. fluorescens is of 100% tolerance to all heavy metals tested except for chromium. However, the extent of growth was affected by increasing heavy metal concentrations for all metals tested implying increased sensitivity to higher metal concentrations. Apart from chromium the heavy metal tolerance of the PGPR P. fluorescens strain indicates that they could survive and have bioremediation potential for HM-polluted environments. Tolerance to heavy metals by certain PGPR microorganisms indicates their suitability as bioinoculants to be applied to contaminated environments in terms of their probable survival. Based on the demonstrated heavy metal tolerance PGPR P. fluorescens strain should be further investigated for the possibility of being used for soil bioremediation purposes in heavy metal contaminated soils.
CONCLUSION
The success of using microbial agents as bioinoculants for soil bioremediation technologies will depend on our ability to manage the rhizosphere to enhance survival and competitiveness of these beneficial microorganisms. The use of commercially available PGPR P. fluorescens as a bioinoculant for the bioaugmentation and bioremediation of contaminated soils relies on its ability to survive, replicate and perform the desired biotechnological appointment. Rhizosphere competence, abiotic stress and heavy metal tolerance to certain common environmental parameters associated with mine lands and/or contaminated soils enables the survivability of the microbial inoculant to be gauged. This study showed that common, widely applied and available agronomic biofertilizers, P. fluorescens, exhibits an exceptional capacity to withstand hostile environmental conditions which in addition to its numerous plant growth promoting traits, make it a desirable microorganism using in environmental biotechnologies applied to ecosystem restoration.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Adams GO, Fufeyin PT, Okoro SE, Ehinomen I (2015). Bioremediation, Biostimulation and Bioaugmention: A Review. International Journal of Environmental Bioremediation and Biodegradation 3(1):28-39. |
|
|
Ahmad S, Lee SY, Kong HG, Jo EJ, Choi HK, Khan R, Lee SW (2016). Genetic determinants for pyomelanin production and its protective effect against oxidative stress in Ralstonia solanacearum. PloS One 11(8):e0160845. |
|
|
Barathi S, Vasudevan N (2001). Utilization of petroleum hydrocarbons by Pseudomonas fluorescens isolated from a petroleum-contaminated soil. Environment International 26(5-6):413-416. |
|
|
Bento FM, Camargo FAO, Okeke BC, Frankenberger WT (2005). Comparative bioremediation of soils contaminated with diesel oil by natural attenuation, biostimulation and bioaugmentation. Bioresource Technology 96(9):1049-1055. |
|
|
Berg G (2009). Plant-microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Applied microbiology and Biotechnology 84(1):11-18. |
|
|
Bruno LB, Karthik C, Ma Y, Kadirvelu K, Freitas H, Rajkumar M (2020). Amelioration of chromium and heat stresses in Sorghum bicolor by Cr6+ reducing-thermotolerant plant growth promoting bacteria. Chemosphere 244:125521. |
|
|
Chaudhary T, Dixit M, Gera R, Shukla AK, Prakash A, Gupta G, Shukla P (2020). Techniques for improving formulations of bioinoculants. 3 Biotech 10(5):1-9. |
|
|
Cornelis P (2010). Iron uptake and metabolism in pseudomonads. Applied Microbiology and Biotechnology 86(6):1637-1645. |
|
|
David BV, Chandrasehar G, Selvam PN (2018). Chapter 10-Pseudomonas fluorescens: A plant-growth-promoting rhizobacterium (PGPR) with potential role in biocontrol of pests of crops. In Crop Improvement through Microbial Biotechnology; Prasad R, Gill SS, Tuteja N, Eds.; Elsevier: Amsterdam, The Netherlands pp. 221-243. ISBN 978-0-444-63987-5. |
|
|
Gavrilescu M (2010). Environmental biotechnology: achievements, opportunities and challenges. Dynamic Biochemistry, Process Biotechnology and Molecular Biology 4(1):1-36. |
|
|
Goddard VJ, Bailey MJ, Darrah P, Lilley AK, Thompson IP (2001). Monitoring temporal and spatial variation in rhizosphere bacterial population diversity: A community approach for the improved selection of rhizosphere competent bacteria. Plant and Soil 232(1):181-193. |
|
|
Goldberg JB (2000). Pseudomonas: Global bacteria. Trends in Microbiology 8(2):55-57. |
|
|
Goswami M, Deka S (2020). Plant growth-promoting rhizobacteria-Alleviators of abiotic stresses in soil: A review. Pedosphere 30(1):40-61. |
|
|
Hider RC, Kong X (2010). Chemistry and biology of siderophores. Natural Product Reports 27(5):637-657. |
|
|
Iqbal M, Edyvean RGJ (2004). Biosorption of lead, copper and zinc on loofa sponge immobilized biomass of Phanerochaete chrysosporium. Minerals Engineering 17(2):217-223. |
|
|
Janek T, Lukaszewicz M, Rezanka T, Krasowska A (2010). Isolation and characterization of two new lipopeptide biosurfactants produced by Pseudomonas fluorescens BD5 isolated from water from the Arctic Archipelago of Svalbard. Bioresource Technology 101(15):6118-6123. |
|
|
Jeyasingh J, Philip L (2005). Bioremediation of chromium contaminated soil: optimization of operating parameters under laboratory conditions. Journal of Hazardous Materials 118(1-3):113-120. |
|
|
Juhasz AL, Naidu R (2000). Bioremediation of high molecular weight polycyclic aromatic hydrocarbons: a review of the microbial degradation of benzopyrene. International Biodeterioration & Biodegradation 45(1-2):57-88. |
|
|
Khan N, Ali S, Tariq H, Latif S, Yasmin H, Mehmood A, Shahid MA (2020). Water conservation and plant survival strategies of rhizobacteria under drought stress. Agronomy 10(11):1683. |
|
|
Kloepper J, Ryu C, Zhang S (2004). Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94(11):1259-1266. |
|
|
Kumar VSK, Shahi S, Singh S (2018). Bioremediation: an eco-sustainable approach for restoration of contaminated sites. Microbial bioprospecting for sustainable development. Springer 115-136. |
|
|
Kraemer SM (2004). Iron oxide dissolution and solubility in the presence of siderophores. Aquatic Sciences 66(1):3-18. |
|
|
Mrozik A, Piotrowska-Seget Z (2010). Bioaugmentation as a strategy for cleaning up soils contaminated with aromatic compounds. Microbiological Research 165(5):363-75. |
|
|
Nadeem SM, Naveed M, Ayyub M, Khan MY, Ahmad M, Zahir ZA (2016). Potential, limitations and future prospects of Pseudomonas spp. for sustainable agriculture and environment: A Review. Soil & Environment 35(2):106-145. |
|
|
Nazli F, Mustafa A, Ahmad M, Hussain A, Jamil M, Wang X, Shakeel Q, Imtiaz M, Esawi MAE (2020). A review on practical application and potentials of phytohormone-producing plant growth-promoting rhizobacteria for inducing heavy metal tolerance in crops. Sustainability 12(21):9056. |
|
|
Palleroni NJ, Cornelis P (2008). The road to the taxonomy of Pseudomonas. Pseudomonas: Genomics and Molecular Biology, Caister Academic Press, UK pp.1-18. |
|
|
Palleroni NJ (2010). Pseudomonas. Topley and Wilson's Microbiology and Microbial Infections, John Wiley & Sons, Limited. |
|
|
Raaijmakers JM, Vlami M, de Souza JT (2002). Antibiotic production by bacterial biocontrol agents. Antonie van Leeuwenhoek 81(1-4):537-547. |
|
|
Rizvi A, Ahmed B, Khan MS, El-Beltagi HS, Umar S, Lee J (2022). Bioprospecting Plant Growth Promoting Rhizobacteria for Enhancing the Biological Properties and Phytochemical Composition of Medicinally Important Crops. Molecules 27(4):1-31. |
|
|
Robertson M, Hapca SM, Moshynets O, Spiers AJ (2013). Air-liquid interface biofilm formation by psychrotrophic pseudomonads recovered from spoilt meat. Antonie Van Leeuwenhoek 103(1):251-259. |
|
|
Roca C, Olsson L (2001). Dynamic responses of Pseudomonas fluorescens DF57 to nitrogen or carbon source addition. Journal of Biotechnology 86(1):39-50. |
|
|
Sánchez-Contreras M, Mart?n M, Villacieros M, O'Gara F, Bonilla I, Rivilla R (2002). Phenotypic Selection and Phase Variation Occur during Alfalfa Root Colonization by Pseudomonas fluorescens F113. Journal of Bacteriology 184(6):1587-1596. |
|
|
Santoyo G, Urtis-Flores CA, Loeza-Lara PD, Orozco-Mosqueda MdC, Glick BR (2021). Rhizosphere Colonization Determinants by Plant Growth-Promoting Rhizobacteria (PGPR). Biology 10(6):475. |
|
|
Shinwari KI, Shah A, Afridi MI, Zeeshan M, Hussain H, Hussain J, Ahmad O, Jamil M (2015). Application of plant growth promoting rhizobacteria in bioremediation of heavy metal Polluted Soil. Asian Journal of Multidisciplinary Studies 3(4):179-185. |
|
|
Soderholm P (2020). The green economy transition: the challenges of technological change for sustainability. Sustainable Earth 3(1):1-11. |
|
|
Sousa AM, Machado I, Nicolau A, Pereira MO (2013). Improvements on colony morphology identification towards bacterial profiling. Journal of Microbiological Methods 95(3):327-335. |
|
|
Thompson IP, Van der Gast CJ, Ciric L, Singer AC (2005). Bioaugmentation for bioremediation: the challenge of strain selection. Environmental Microbiology 7(7):909-915. |
|
|
Van den Broek D, Bloemberg GV, Lugtenberg B (2005). The role of phenotypic variation in rhizosphere Pseudomonas bacteria. Environmental Microbiology 7(11):1686-1697. |
|
|
Weller D (2007). Pseudomonas biocontrol agents of soil-borne pathogens: looking back over 30 Years. Phytopathology 97(2):250-256. |
|
|
Whipps JM (2001). Microbial interactions and biocontrol in the rhizosphere. Journal of Experimental Botany 52(suppl_1):487-511. |
|
|
Wu CH, Wood TK, Mulchandani A, Chen W (2006). Engineering plant-microbe symbiosis for rhizoremediation of heavy metals. Applied and Environmental Microbiology 72(2):1129-1134. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0