ABSTRACT
Detarium microcarpum Guill and Perr is a multipurpose tree species indigenous to semi-arid regions of Sub-Saharan Africa. It is being exploited to local extinction due to high dependence for fuelwood and other uses. The present study explored different pre-treatment methods for enhancing seed germination and growth of D. microcarpum in the Guinea savanna zone of Ghana. The experiment employed a 4 × 4 factorial design with seeds subjected to four pre-treatments (50% sulphuric acid concentration, 98% sulphuric acid concentration, cold water and hot water) at four pre-treatment time durations. Percentage germination varied significantly between pre-treatments (p < 0.05) with cold water treatment recording the highest mean germination (73.06%) and the 98% sulphuric acid concentration recording the least (47.72%). Germination rate had a moderate positive relationship with plant height (rxy = 0.49) and collar diameter (rxy = 0.54). The study recommends seed immersion in cold water for 48 h as the most efficient pre-treatment for D. microcarpum.
Key words: Detarium microcarpum, germination percentage, plant height, pre-treatment.
Detarium microcarpum is a perennial woody plant indigenous to the semi-arid regions of Sub Saharan Africa which occurs predominantly in Benin, Burkina Faso, Cameroon, Central African Republic, Ghana, Guinea, Guinea Bissau, Niger, Nigeria, Senegal and Togo (Oibiokpa et al., 2014). There are two reported species of the genus with D. senegalensis growing in riparian and dry forests areas, whilst D. microcarpum grows in dry savannas (Tropical Plant Database, 2019). D. microcarpum thrives in a wide variety of soils including degraded and rocky areas with annual rainfall of about 600-1000 mm (Abreu et al., 1999). Although it is commonly found in fallow lands and wild bushes, it is sometimes retained on farmlands for soil improvement, fuelwood, food and medicinal purposes (Oibiokpa et al., 2014).
According to FAO (1995), D. microcarpum is a leguminous tree species which improves soil fertility when retained on farmlands through nitrogen fixation and leaf litter decomposition. The edible fruits of D. microcarpum are consumed by human and wild animals in regions where the species is found (Akpata and Miachi, 2001).
The fruit flour is reported to contain about 42% carbohydrates, 36% lipids and 11% protein (Anhwange et al., 2004). The fruit pulp is rich in minerals and essential
vitamins such as vitamin C, E, B2 and folic acid which which serve as a major food supplement during the dry season (Oibiokpa et al., 2014). These nutritional properties highlight the potential contribution of D. microcarpum to food security in Africa. The fruits are equally sold in local markets and contribute to economic empowerment in rural communities (Akpata and Miachi, 2001).
Moreover, D. microcarpum is used in traditional medicine for the treatment of various ailments including tuberculosis, meningitis and diarrhea due to its antimicrobial properties (Abreu et al., 1998). The seed coat is also reported to possess antimicrobial activity which could be used in the control of infectious diseases (Ebi and Afieroho, 2011). It also serves as a major fuelwood species with charcoal produced from the wood delivering about 1968 KJ/kg of caloric power (Kabore et al., 2005) and ranked among the most preferred fuelwood species in its naturally growing areas (Sawadogo, 2007).
The multipurpose uses have resulted in overexploitation of the species to local extinction in some areas (Kabore, 2005) mainly due to the high dependence on wild plant sources with little attention on domestication of the species. However, effective domestication will require knowledge on regeneration and other aspects of the plant biology (Bohra et al., 2018). Seed germination is known to be an important step towards raising a successful crop stand (Finch-Savage and Bassel, 2016), but there is a paucity of information on pre-treatment methods for D. microcarpum seeds. Kouyate and Van Damme (2006) recommended immersion of seeds in sulphuric acid as a good pre-treatment for D. microcarpum seeds but their study was not explicit on the recommended acid concentration and best duration of seed immersion in acid. The present study examined the percentage germination and initial growth performance of D. microcarpum using different pre-treatment methods.
Study area
The experiment was conducted at the tree nursery of the Faculty of Natural Resources and Environment of the University for Development Studies, Ghana. It lies between latitude 9° 25' N to 10° 40' N and longitude 0° 58' N to 1° 12' W (Figure 1). The area records a monomodal rainfall pattern with a mean annual rainfall of 1127 mm. Mean monthly minimum and maximum temperatures are 26.6 and 35.6°C respectively, with a mean annual temperature of 29.7°C (SARI, 2016). The vegetation is dominantly grassland interspersed with indigenous and exotic tree species such as Parkia biglobosa (dawadawa), Vitellaria paradoxa (shea), Lannea acida (lanea), Azardirachta indica (neem), Magnifera indica (mango), Tectona grandis (teak), Senna siamea (cassia). Seed boxes were kept in a shade net with average light intensity of 11.19 Lux.
Seed collection and viability test
Fruits of D. microcarpum were collected from fallow lands of Nandom community in Upper West region of Ghana. Collected fruits were initially sorted to eliminate diseased and bruised fruits. The fruits were cracked to remove seeds for pre-treatment. Prior to the germination experiment, 50 seeds were sampled for a seed viability test using floating test.
Experimental design
Seeds were subjected to four pre-treatment methods at four pre-treatment time durations with the untreated seeds as control. The pre-treatment methods were;
(i) Seeds soaked in 98% sulphuric acid concentration for 1, 5, 10 and 15 min
(ii) Seeds soaked in 50% sulphuric acid concentration for 1, 5, 10 and 15 min
(iii) Seeds soaked in cold water for 12, 24, 36, and 48 h
(iv) Seeds soaked in boiled water (100°C) for 12, 24, 36, and 48 h
(v) Control (untreated seeds)
Each treatment combination had 45 seeds, making a total of 765 seeds for the experiment. The pre-treated seeds were sown in seed boxes (50 cm × 15 cm × 10 cm) half filled with topsoil. The seed boxes were arranged in a Completely Randomized Design with 15 seeds per box. Each treatment combination was replicated in three seed boxes. Seed boxes were watered twice daily with 1500 ml of water per box. Weeds were controlled by hand to prevent competition.
Data collection
Data was collected on seed emergence, plant height, collar diameter and root length. Number of seeds emerged was recorded daily per seed box from the day of first germination to the end of the germination period (4th week after sowing). Growth parameters were recorded once every two weeks from the 4th week after sowing to the 10th week after sowing. Seedlings were uprooted after the tenth week for root length measurement. Plant height and root length were measured with a measuring tape whilst a caliper was used for collar diameter.
Data analysis
Percentage seed Germination (PG) and Germination Rate (GR) were estimated with following equations:
Where, PG is Percentage Germination and T is Time taken in days.
Data set on seedling emergence proportions were arcsine-square root transformed before each factor was subjected to Analysis of Variance (ANOVA). Treatment means were separated with Bonferroni corrections at 95% confidence level. Pearson’s correlation coefficient was used in establishing relationship between germination rate and plant size (plant height, collar diameter and root length). All analysis was done with Genstat 18th edition.
Effect of pre-treatment methods on germination of D. microcarpum
Percentage seed germination varied significantly between pre-treatment methods (P < 0.05) with cold water treatment recording the highest mean percentage germination of 73.06% while seed soaking in 98% sulphuric acid resulted in the lowest germination (46.72%). Amongst sulphuric acid treatments, seed soaking in 50% sulphuric acid recorded higher germination (53.22%) when compared with seed soaking in 98% sulphuric acid (46.72%) (Figure 2).
Aside the general significant effect of pre-treatment on seed germination, the duration of seed immersion also had significant effect within treatments. For instance, percentage seed germination differed significantly between the four time durations of seed immersion in cold water with 48 h recording the highest germination of 86% (Table 1). Again, seeds immersed in 50% sulphuric acid concentration for 15 min had significantly higher germination percentage (68%) when compared with other time durations of immersion in 50% sulphuric acid. Pre-treatment method also influenced number of days to seed emergence. Seeds immersed in cold water for 48 h germinated earlier (8 days), whereas untreated seeds took longest time (12 days) to first seed germination.
The cold water treatment recording the highest percentage as well as shortest time to first seed emergence could be attributed to the ability of cold water to enhance seed coat permeability. This enabled gaseous exchange and enzymatic hydrolysis to transform the embryo into a seedling without negatively affecting the functional organs of the seed (Olatunji et al., 2013). This also agrees with Azad et al. (2011) who identified water as a necessary requirement for seed germination. D. microcarpum has a hard seed coat which needs to be ruptured before radicle and plumule emergence. Hence seeds that were immersed in cold water for a longer period (48 h) had an early seed coat rupture and permeability which facilitated a faster rate of emergence. This could explain the significantly higher percentage germination and early days to emergence recorded among seeds that were immersed in cold water for 48 h. This phenomenon confirms earlier reports by Missanjo et al. (2013) and Mwase and Mvula (2011), who reported seed coat permeability as one of the determinants of seed germination.
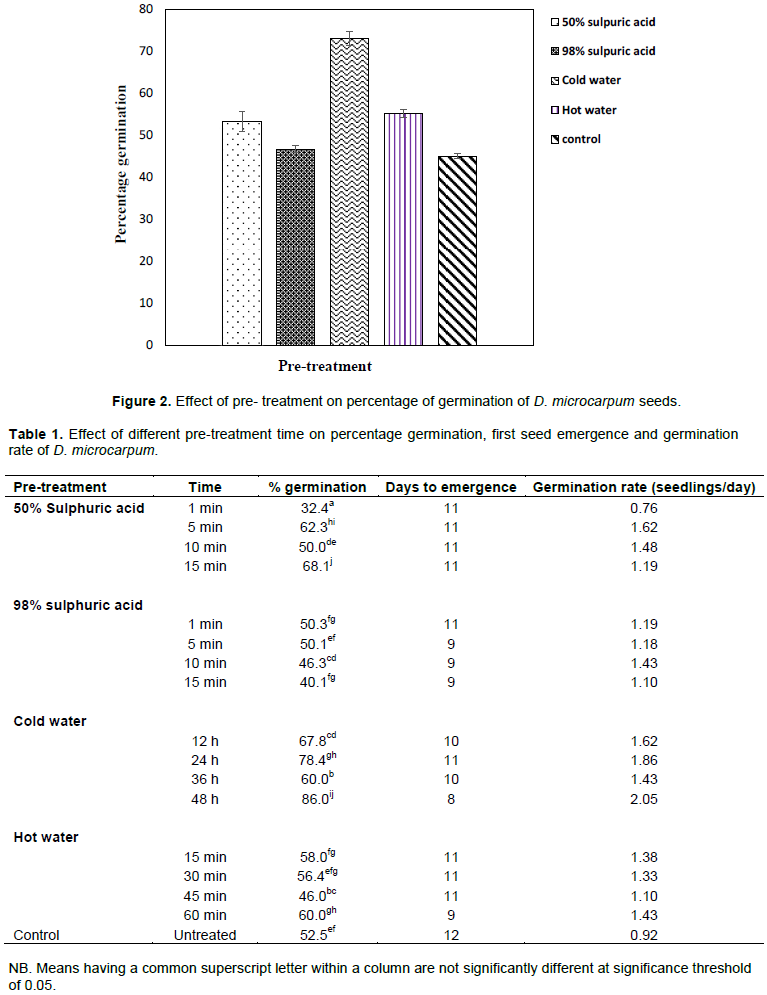
The relatively low percentage germination in 98% sulphuric acid as compared to control (Figure 2) seem to suggest detrimental effect of this chemical to D. microcarpum seeds at higher concentrations. This is in accordance with Asl et al. (2011), that sulphuric acid has a detrimental effect on seed embryo. This could be attributed to the fact that some enzymes have specific pH ranges, therefore higher acid concentration above this range tends to provide unfavourable pH conditions for normal enzymatic activity. However, at low concentrations, sulphuric acid could have a positive effect on seed germination; this was evident in the fact that 50% sulphuric acid had a higher percentage germination than the control treatment (Figure 2).
Time of immersion in sulphuric acid also had an effect on seed germination with germination percentage decreasing with increasing time duration of seed immersion in 98% sulphuric acid concentration (Table 1). However, in 50% sulphuric acid concentration percentage germination generally increased with increasing time of immersion. The effect of time duration of treatment on germination is not limited to D. microcarpum. This positive effect of time duration of pre-treatment on seed germination was equally reported in Tamarindus indica (Abubakar and Muhammad, 2013; Muhammad and Amusa, 2003).
Hot water pre-treatment resulted in a low germination percentage as compared to the cold water treatment probably due to the high temperature the seeds were exposed. This argument is supported by the findings of Singh et al. (2019) who indicated that hot water may tend to be detrimental to enzymatic activities at higher temperatures when used as pre-treatment. The higher percentage germination recorded in hot water treatment as compared to 50% sulphuric acid treatment is dissimilar to the response of T. indica to the same pre-treatments. T. indica seeds immersed in 50% concentrated sulphuric acid recorded a higher germination percentage than seeds that were immersed in hot water (Abubakar and Muhammad, 2013). Again, Adonsonia digitata seeds rather recorded a higher percentage germination in 80% sulphuric acid concentration as compared to cold water treatment for 72 h (Oboho and Ahanon, 2017). This variation in tropical plant species response to pre-treatments points out differences in the inhibitory factors to germination between plants.
The control treatment generally recorded low percentage germination as compared to most pre-treatments (Cold water, 50% sulphuric acid and hot water) which could be an indication of some level of dormancy in D. microcarpum. This suggests that pre-treatments have positive influence on germination of D. microcarpum which could be a boon to nursery managers and foresters for the domestication of the species.
Relationship between germination rate and growth of D. microcarpum seedlings
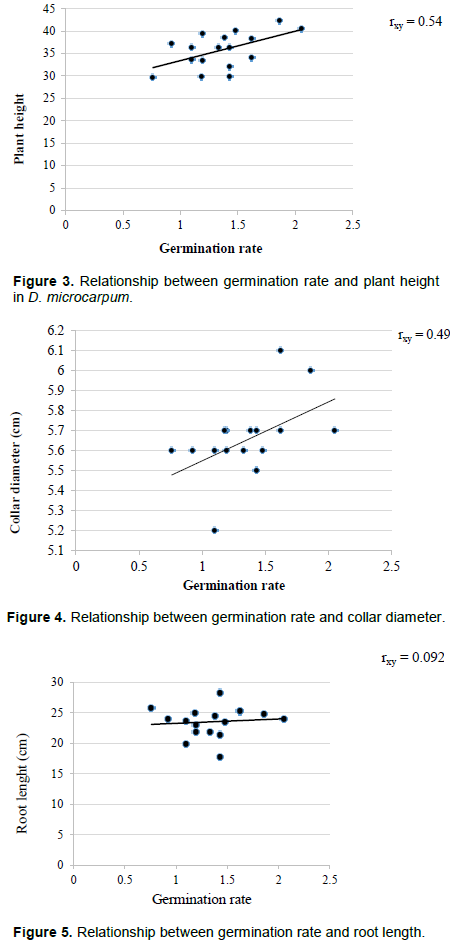
Germination rate correlated positively with plant growth parameters recording moderate positive relationships with plant height (rxy = 0.52) and collar diameter (rxy = 0.49) (Figures 3 and 4). However, root length had a weak positive relationship with germination rate (rxy= 0.092). El-Bably and Rashed (2018) have reported positive effects of pre-treatment on growth of Adansonia digitata seedlings. The positive relationship between germination rate and seedling growth performance suggests that, effect of pre-treatment on germination of D. microcarpum also translates into seedling size. This is similar to the findings of Chubamerenla et al. (2015) who reported a significant variation in growth of Delonix regia seedlings that emerged from different pre-treatments. This equally reveals an indirect influence of pre-treatment on growth performance of seedlings. Therefore, the positive relationship between germination rate and growth parameters of D. microcarpum seedlings implies that, benefits of pre-treatment does not end in germination but also contribute to the survival and establishment of seedlings in the field (Figure 5).
The study concludes that both pre-treatment method and duration of treatment have significant effects on the germination and growth performance of D. microcarpum. Soaking D. microcarpum seeds in cold water for 48 h could be recommended for large scale production of seedlings as it resulted into 86% germination. Although acid treatment can equally enhance germination, higher acid concentration could result in detrimental effects. Similarly, cold water treatment resulted in an early germination of D. microcarpum seed as compared to hot water pre-treatments. Significant effect of pre-treatment on germination translated into a positive effect on seedling size with plant height, collar diameter and root length all positively correlated with germination. The study therefore recommends cold water as an effective pre-sowing treatment for D. microcarpum.
The authors have not declared any conflict of interests.
REFERENCES
|
Abreu PM, Martins ES, Kayser O, Bindseil KU, Siems K, Seemann A, Frevert J (1999). Anti-microbial, anti-tumor and anti-leishmania screening of medicinal plants from Guinea-Bissau. Phytomedicine 6(3):187-195.
Crossref
|
|
|
|
Abreu PM, Rosa VS, Araujo EM, Canda AB, Kayser O, Bindseii KV, Siems K, Seeman A (1998). Phytochemical analysis and antimicrobial evaluation of Detarium microcarpum bark. Pharmaceutical and Pharmacological Letters 8:107-111.
|
|
|
|
|
Abubakar ZA, Muhammad A (2013). Breaking Seed Dormancy in Tamarind (Tamarindus indica) A Case Study of Gombe Local Government Area. Journal of Applied Sciences and Environmental Management 17 (1):83-87.
|
|
|
|
|
Akpata MI, Miachi OE (2001) Proximate composition and selected functional properties of Detarium microcarpum. Plant Foods for Human Nutrition 56(4):297-302.
Crossref
|
|
|
|
|
Anhwange BA, Ajibola VO, Oniye SJ (2004). Chemical studies of the seeds of Moringa oleifera (Lam) and Detarium microcarpum (Guill and Perr) Journal of Biological Sciences 4(6):711-715.
Crossref
|
|
|
|
|
Asl MB, Sharivivash R, Rahbari A (2011). Effect of Different Treatments on Seed Germination of Honey Locust (Gleditschia triacanthos). Modern Applied Science 5(1):200-204.
Crossref
|
|
|
|
|
Azad MS, Manik MR, Hasan MS, Matin MA (2011). Effect of different pre-sowing treatments on seed germination percentage and growth performance of Acacia auriculiformis. Journal of Forestry Research 22(2):183-188.
Crossref
|
|
|
|
|
Bohra P, Waman AA, Basantia D, Devi HL, and Reang E (2018) Domestication and conservation efforts in Haematocarpus validus (Miers.) Bakh. F. ex Forman: an underutilized fruit species and natural colourant. Current Science 115(6):1098-1105.
Crossref
|
|
|
|
|
Chubamerenla I, Somnath S, Hemant K, Josiah KM (2015). Effect of Different Pretreatment Method on Seed Germination of Gulmohar (Delonix regia). Trends in Biosciences 8(19):5105-5110.
|
|
|
|
|
Ebi GC, Afieroho OE (2011). Phytochemical and antimicrobial studies on Detarium microcarpum Guill and Sperr (Caesalpinioceae) seeds Coat. African Journal of Biotechnology 10(3):457-462.
|
|
|
|
|
El-Bably SMZ, Rashed NM (2018). Influence of pre germination treatments on overcoming seed dormancy and seedling growth of baobab (Adansonia digitata L.). Zagazig. Journal of Agricultural Research 45(2):465-476.
|
|
|
|
|
FAO (1995). State of the World's Forests - 1995. Rome, Italy: Food and Agriculture Organization of the United Nations.
|
|
|
|
|
Finch-Savage WE, Bassel GW (2016). Seed vigour and crop establishment: extending performance beyond adaptation. Journal of Experimental Botany 67(3):567-591.
Crossref
|
|
|
|
|
Kabore C (2005). Aménagement des forêts au Sahel - Point sur vingt années de pratiques au Burkina Faso. Ouagadougou, Burkina Faso: Ministère de l'Environnement et de l'Eau.
|
|
|
|
|
Kouyate AM, van Damme P (2006). Detarium microcarpum Guill. & Perr. In: Schmelzer, G.H. & Gurib-Fakim, A. (Eds.) Medicinal plants 1 [CD-Rom]. Wageningen, Netherlands: PROTA Foundation 11(1).
|
|
|
|
|
Missanjo EMC, Kapira D, Banda H, Kamanga- Thole G (2013). Effect of seed size and pretreatment methods on germination of Albizia lebbeck. ISRN Botany ID 969026, 4.
Crossref
|
|
|
|
|
Muhammad S, Amusa NA (2003). Effects of sulphuric acid and hot water treatment on seed germination of tamarind. African Journal of Biotechnology 2:276-279.
Crossref
|
|
|
|
|
Mwase F, Mvula T (2011). Effect of seed size and pre-treatment methods of Bauhinia thonningii Schum on germination and seedling growth. African Journal of Biotechnology 10 (26):5143-5148.
|
|
|
|
|
Oboho EG, Ahanon EC (2017). Effect of different pre-treatments on seed germination and watering regime on growth of Adansonia digitata (Linn.) seedlings. Asian Journal of Science and Technology 8(4):4569-4573.
|
|
|
|
|
Oibiokpa IF, Adoga IG, Saidu AN, Shittu OK (2014). Nutritional composition of Detarium microcarpum fruit. African Journal of Food Science 8(6):342-350.
Crossref
|
|
|
|
|
Olatunji D, Maku JO, Odumefun OP (2013). The effect of pretreatments on the germination and early seedlings growth of Acacia auriculiformis Cunn. Ex. Benth. African Journal of Plant Science 7:325-330.
|
|
|
|
|
SARI (Savannah Agricultural Research Institute) (2016). Annual Report for the year 2016. Nyankpala.
|
|
|
|
|
Sawadogo L (2007). Etat de la biodiversité et de la production des ligneux du chantier d'amenagement forestier du Nazinon après une vingtaine d'année de pratiques d'aménagement. Bogor, Indonesia: Center for International Forestry Research. (CIFOR). ISBN ISBN: 978-979-1412-27-8.
|
|
|
|
|
Singh S, Bharat NK, Singh H, Kumar S, Jakhar S, Vijay (2019). Effect of hot water treatment of seeds on seed quality parameters and seedling growth parameters in bell pepper (Capsicum annuum) Indian Journal of Agricultural Sciences 89 (1):133-137.
|
|
|
|
|
Tropical Plants Database (2019). Ken Fern. tropical.theferns.info. <tropical.theferns.info/viewtropical.php?id=Detarium+macrocarpum. Accessed on 14th May, 2019.
|
|