Full Length Research Paper
ABSTRACT
It is important to always monitor the bioaccumulation potential for heavy metals by organisms especially the edible ones, to assess their potential risk to human health. This study evaluated the bioaccumulation of heavy metals in the shell and soft tissues of snails. Forty snails each were purchased from Ikire and Ore towns. The snails’ shells, feet, digestive tracts and glands were analysed for bioaccumulation of heavy metals using an Atomic Absorption Spectrophotometer. The results showed that the concentration of heavy metals varied with the location and species of the snail. Archachatina marginata from Ore accumulated higher concentrations of heavy metals than A. marginata from Ikire. The concentration of Pb in Achatina achatina and A. marginata from Ikire, and Cd in A. marginata from Ore are slightly above the FAO/WHO permissible limits. Foot bioaccumulated more heavy metals in A. achatina while the digestive gland bioaccumulated more heavy metals in A. marginata. The study concluded that the shell and soft tissues of A. achatina at Ikire and A. marginata at Ikire and Ore bioaccumulated some levels of toxic heavy metals. A. achatina and A. marginata are capable of being used as a sentinel to study the physiological and biochemical imbalances in living organisms arising from the accumulation of heavy metals.
Key words: Bioaccumulation, heavy metal pollution, snails, Achatina achatina, Archachatina marginata.
INTRODUCTION
Giant African land snail is the common name for Achatina achatina (Linnaeus). It can grow up to 200 mm in length and a maximum diameter of 100 mm in the native range within the northern part of West Africa (Dar et al., 2017). Archachatina marginata (Swainson) is also one of the giant African snails with the common name banana rasp, it has the potential to grow up to 210 mm in length and 130 mm in diameter (Awodiran et al., 2012). A. marginata native range is within West Africa (Barker, 2001). These two species belong to the family Achatinidae.
Achatinids are generally nocturnal forest dwellers but can fit into disturbed habitats. Hence, they are active more during the period of high humidity and feed on a wide range of living and dead plant materials. When reared in captivity, food materials often consumed by this species include banana, lettuce, papaya and the rind of watermelon (Ajayi and Babatunde, 2022). Achatinids attain sexual maturity at 9 to 10 months and can live up to 5 years (Cowie et al., 2009).
Appenroth (2010) and Oguh et al. (2019a) described heavy metals as those metals whose atomic mass exceeds that of Calcium; having relative densities greater than 5 g/cm3. Heavy metals are toxic even at low concentrations, not biodegradable but can be assimilated and bioaccumulated in the tissues (Gupta and Singh, 2011). Heavy metals may include some trace elements such as Zinc (Zn), Iron (Fe), and Nickel (Ni) that are nutritionally required for enzymatic reactions and have functional roles in various metabolic processes. They become toxic when their concentrations exceed a certain limit (Gawad, 2018). The other category of heavy metals is nonessential and they are environmental pollutants. They are toxic even at a very low concentration. Examples include Arsenic (As), Cadmium (Cd), Thallium (Ti) and Tin (Sn).
The two major sources of heavy metals are natural and anthropogenic. Natural sources include atmospheric sources, geological weathering, the earth’s crust and volcanic emissions while anthropogenic sources are a result of various human activities through effluent from automobiles, weathering, sewage sludge, fossil fuel burning, manufacturing industries, and fertilizer application (Mahmoud and Abu-Taleb, 2013). The probable health hazards posed by heavy metals remain a global concern especially, in developing countries, where treatment and elimination of effluents are inadequate or non-existent (Banaee and Taheri, 2019).
Snail meat is proteinous, rich in essential fatty acids and amino acids, supplies enough essential minerals, and contains less fat and cholesterol (Ademolu et al., 2004). Thus, snail meat holds the potential to bridge the gap of would-be nutritional deficiency owing to its nutritional profile, palatability and availability (Ajayi and Babatunde, 2022). These potentials are being harnessed because snails are easily accessible either by production, purchase or picking (hunting) in the wild (Anthony et al., 2010; Adeniyi et al., 2013).
Though snails are omnivores (Amobi and Ezewudo, 2019), wild snails which are commonly found in the bush, have free access to soil, vegetables, fruits, and plants which might have grown in heavy metals contaminated areas (Nica et al., 2012; Louzon et al., 2021). Domesticated snails fed with plant food materials that have been contaminated can accumulate such heavy metals. This could adversely affect their growth and reproductive capacity. Plants that grow near the roadside, domestic and industrial waste dumpsites tend to absorb and accumulate heavy metals (Singh et al., 2012; Salih et al., 2021). It is worthy to note that some of these elements are essential for the normal functions of the body but could cause acute and chronic poisoning when their concentrations exceed the tolerable limit.
Incessant consumption of fruits and vegetables grown in heavy metal highly contaminated soils and eating of animals that feed on the plants grown on such soil are the main route through which man gets infected (Khan et al., 2014). Heavy metals can bioaccumulate in the tissues of humans and non-humans and wreck great health havoc. Metal-induced pathologies remain a global public health concern (Hina et al., 2011; Izah et al., 2017). The toxic effects of heavy metals may be due to their interference with normal body biochemistry in the normal metabolic process (Okunola et al., 2011). Metals for instance Pb, Cd, and As may cause toxicity by preferentially interacting with thiol-containing groups of biomolecules, oxygen, or sulphur-containing compounds to induce oxidative stress, causing tissue damage (Lemire et al., 2013). Heavy metals are known disruptors of lipid homeostasis and the antioxidant system such as Pb and As in rats (Ademuyiwa et al., 2010), Cd in crabs (Yang et al., 2013), and Cd and Pb in snails and fish (Banaee and Taheri, 2019; Sarah et al., 2019). Exposure to Pb may cause mitochondrial apoptosis (Jin et al., 2017), disrupt the cellular redox state, inhibit haeme biosynthesis (Mani et al., 2018), and cause convulsion, encephalopathy and hypertension (Iweala et al., 2014). Cadmium has been reported to be hepatotoxic and nephrotoxic (Iweala et al., 2014). Cd may disrupt the metabolism of lipids by altering the levels of triacylglycerol, cholesterol, and lipoproteins via the inhibition of lipogenic enzymes (Yang et al., 2013). Keratosis, mitochondrial damage, disruption of glycolysis, dyslipidaemia, and carcinogenicity are hallmarks of arsenic (As) toxicity (Gupta and Singh, 2011; Afolabi et al., 2015). Furthermore, heavy metals toxicity may lower endogenous antioxidant molecules (such as metallothionein), impede secondary antioxidant enzymes (such as Arylesterase), reduce glutathione levels, increase lipid peroxidation, and induce oxidative stress (Gupta and Singh, 2011; Izah et al., 2017; Banaee and Taheri, 2019).
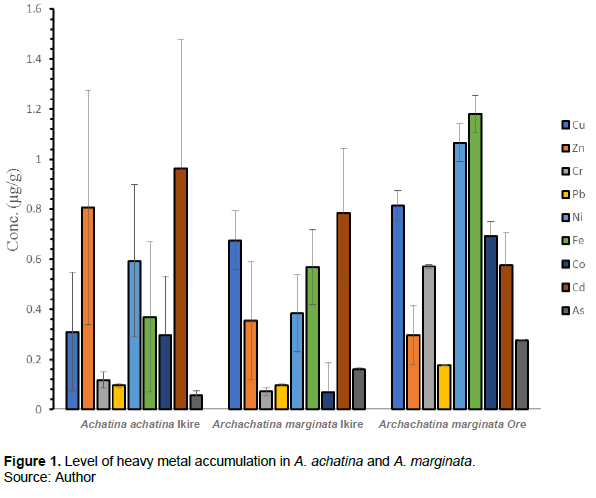
With these dangers posed by heavy metals in mind, it is appropriate to identify organisms that can be used as sentinel in the study of heavy metal pollution in our environment and the level of accumulation of such heavy metals in living organisms along with the physiological and biochemical imbalances arising from the bioaccumulation. This study seeks to evaluate the level of heavy metals (Copper, Zinc, Chromium, Lead, Nickel, Iron, Cobalt, Cadmium and Arsenic) (Figure 1) in selected tissues of A. achatina and A. marginata with the view to compare the extent of heavy metals bioaccumulation in the tissues of the snails from the two sampling locations and compare the levels of heavy metals with established regulatory standards.
MATERIALS AND METHODS
Area of study
Forty snails each were purchased from Ikire and Ore in June and July 2021. The month of June is an active farming season in southwest Nigeria when agrochemicals are used on farmlands for plant protection and weed control.
Ikire town is the administrative headquarter of Irewole local government of Osun State, Nigeria. It is the gateway town into Osun from Oyo State. Ikire is within the basin of River Osun; it lies around latitude 07°21'29'' - 07°24'36'' North and longitude 004°10'11'' - 004°13'43'' East. Ore town is the administrative headquarter of Odigbo local government area in Ondo State, Nigeria. It is the major town separating southwestern Nigeria from the southeast; its geographical coordinates are between 06°42'18'' - 06°46'30'' North and 004°51'18'' - 004°54'55'' East.
Land use
The primary activity in Ikire is farming. The proximity to Ibadan, a major commercial and industrial centre in southwest Nigeria, facilitates the easy movement of goods and services. Ore is a commercial town with agriculture being the mainstay of the economy, cultivating different food crops and cash crops like cassava, plantain, cocoa etc. It is reputable for the large bitumen deposit in Ondo state.
Sample collections
Giant African land snails were purchased from farmers who sourced the snails in the wild. Procured snails were immediately taken into the Physiology laboratory at the Department of Zoology, Obafemi Awolowo University, Ile-Ife for identification. Fifteen A. achatina and twenty-five A. marginata were bought at Ikire; all the forty snail samples from Ore were A. marginata. Only fully grown snails were purchased because this is the preferred size consumed by the local population.
Dissection of snail specimens
Snails were dissected according to the method described by Low et al. (2016). The snails were thoroughly washed with distilled water. The snails were dissected to remove the foot, the digestive gland and the digestive tract. These parts were stored in small plastic jars and preserved in the deep freezer ready to be analysed. Snail shells were also kept in polythene bags and later analysed.
Determination of heavy metals
Each body part was defrosted for 2 h, weighed into a pre-weighed crucible and dried at 80°C in Gallenkamp hot box oven. The sample weights were taken and recorded at 4 h intervals until a constant weight was obtained. The samples were ground separately to fine particles using clean, dried mortar and pestle and then sifted using a sieve of particle size 0.02 mm. Each powdered sample (0.5 g) was measured into a 100 ml beaker; 5 ml of aqua regia HCL and HNO3 (3:1) was added to digest the sample. The samples were evenly distributed in the acid using a glass stirring rod. The digested samples were filtrated (using Whatman filter paper No. 1) into a cylinder. The filtrate was made up to 25 ml of distilled water. The concentration of heavy metals: viz. Arsenic, Cadmium, Chromium, Cobalt, Copper, Iron, Lead, Nickel and Zinc in the samples was examined using PG 990 model Atomic Absorption Spectrophotometer (AAS).
Statistical analysis
Data were analysed using one-way ANOVA and Independent-sample T-test in IBM SPSS version 25. Tukey’s HSD Post Hoc test was used to resolve differences among means. P < 0.05 indicates significant differences among groups.
RESULTS
Concentration (µg/g) of heavy metals in A. achatina procured from Ikire
The mean concentration of heavy metals in A. achatina collected from Ikire is shown in Table 1. Zn, Cr and Fe were accumulated in the shell more than in other organs. The foot accumulated more Cu, Co and Cd than other organs. The digestive tract had the highest concentration of Pb and Ni. The digestive gland accumulated the highest concentration of As and the lowest concentration of Pb and Ni. There was a significant difference (p < 0.005) in the concentration of heavy metals across the organs.
Concentration (µg/g) of heavy metals in A. marginata collected from Ikire
The results summarized in Table 2 showed that in A. marginata collected from Ikire, the shell had the lowest concentration of Pb, Co and Cd while the foot had the highest level of Cr, Pb and As. The digestive tract had the lowest level of Cr. However, Ni in the digestive tract was higher than in the other organs. The digestive gland had the highest concentration of Zn, Fe and Cd and the lowest concentration of Cu and As. The concentration of Cu and Zn in the digestive gland was significantly different (p < 0.05) from the concentration in the shell, foot and digestive tract.
Moreover, there was astatistically significant (p < 0.05) difference in the level of Pb bioaccumulated across the organs.
Concentration (µg/g) of heavy metals in A. marginata collected from Ore
As shown in Table 3, the mean concentrations of Zn, Ni and As were more in the shell than in other tissues whereas the mean concentrations of Cu, Cr and Cd in the shell were lower than in other tissues. The concentration of Fe and Cd in the foot was higher than in other tissues. The concentration of Co in the foot was the least among the tissues. The highest concentration of Cr was recorded in the digestive tract while Pb, Fe and As in the digestive tract were lower than in other tissues. The digestive gland accumulated more Cu and Pb than other tissues while the level of Zn and Ni accumulated in the digestive gland were lower than in other tissues. There was a significant difference in the level of accumulated heavy metals across the tissues (p < 0.005) except Pb.
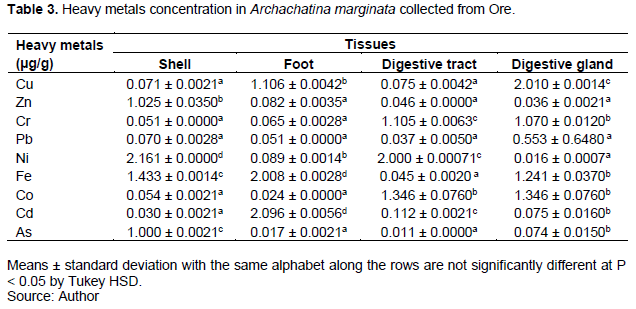
Relationship between the levels of heavy metals in the shell, foot, digestive tract and digestive gland of A. achatina and A. marginata collected from Ikire and Ore
Independent-sample t-test was used to compare the levels of heavy metals in the shell, foot, digestive tract and gland of A. achatina and A. marginata collected from Ikire and Ore. The result revealed that there was a significant difference (p < 0.05) in the level of heavy metals within the shell and foot (except Cu) of A. achatina and A. marginata in Ikire and Ore. Similarly, there was a statistical difference (p < 0.05) in the heavy metal concentrations except for Zn, Pb, Fe, Cd in the digestive tract of A. marginata collected from Ikire and Ore; and Zn and Fe in the digestive tract of A. achatina and A. marginata from Ikire.
Pb, Fe and As in the digestive glands of A. marginata from Ikire and Ore; Cu, Ni and As in the digestive glands of A. achatina and A. marginata from Ikire showed no significant difference (p > 0.05). The level of heavy metals in the shell, foot, digestive tract and digestive gland of A. achatina and A. marginata gathered from Ikire; and A. marginata from Ikire and Ore were not significantly different (p > 0.05).
Overall, the concentration of heavy metals in snail tissues varied with the location and species of the snail. Cd (0.963 µg/g) followed by Zn (0.808 µg/g) were the most accumulated heavy metals and As (0.058 µg/g) had the minimum accumulation in A. achatina from Ikire. The trend of heavy metal in A. achatina from Ikire showed that Cd > Zn > Ni > Fe > Cu > Co > Cr > Pb > As. In A. marginata from Ikire, Cd (0.787 µg/g) and Cu (0.676 µg/g) were the most accumulated heavy metals while Co (0.070 µg/g) was the least accumulated metal. The concentration of metals was detected in the following order Cd > Cu > Fe >Ni > Zn > As > Pb > Cr > Co. However, the trend of heavy metals in A. marginata from Ore revealed that Fe (1.182 µg/g) and Ni (1.066 µg/g) were the most accumulated heavy metals and Pb (0.178 µg/g) had the minimum accumulation following the trend: Fe > Ni > Cu > Co > Cd >Cr > Zn > As > Pb (Fig. 1). Moreover, the trend of heavy metals accumulation in A. achatina from Ikire showed that Foot (0.635 µg/g) > Shell (0.433 µg/g) > Digestive gland (0.320 µg/g) > Digestive tract (0.218 µg/g). The trend of heavy metals in A. marginata from Ikire was Digestive gland (0.508 µg/g) > Digestive tract (0.380 µg/g) > Foot (0.296 µg/g) > Shell (0.224 µg/g) while Digestive gland (0.713 µg/g) > Shell (0.655 µg/g) > Foot (0.615 µg/g) > Digestive tract (0.531 µg/g) was observed in A. marginata from Ore (Figure 2).
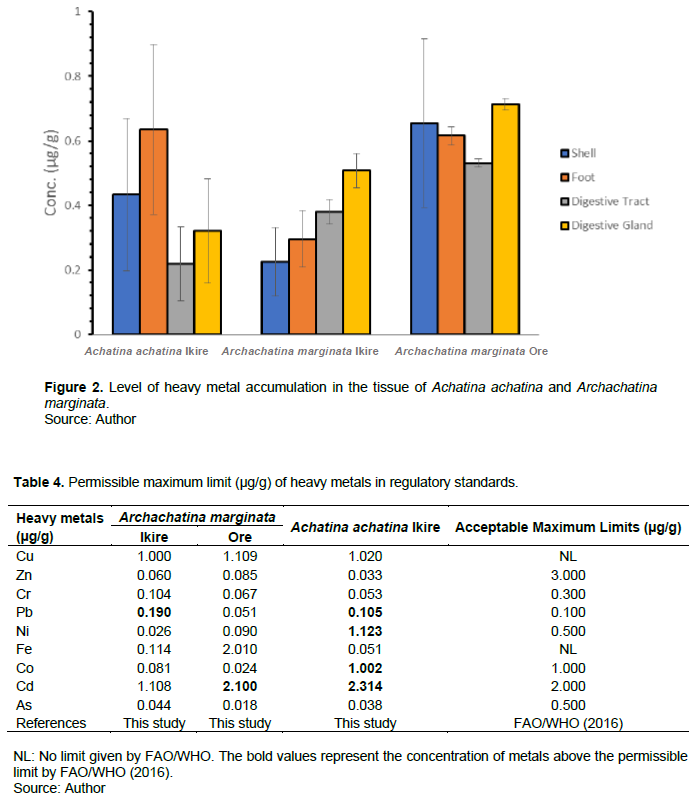
Comparison of inherent heavy metals with regulatory standards
The level of heavy metals in the tissues of A. achatina and A. marginata collected from Ikire and Ore were compared with the established regulatory safety standards for human consumption concerning the edible parts (Table 4). The concentrations of heavy metals recorded in the edible parts of A. achatina and A. marginata are lower than the FAO/WHO (2016) regulatory limits except for Pb (0.105 µg/g), Ni (1.123 µg/g), Co (1.002 µg/g) and Cd (2.314 µg/g) in A. achatina; Pb (0.190 µg/g) in A. marginata from Ikire, and Cd (2.100 µg/g) in A. marginata from Ore which is slightly above the permissible level.
DISCUSSION
Bioaccumulation of heavy metals in tissues varies significantly amongst the taxa and conspecifics (Iwegbue et al., 2009). The concentration of heavy metal in the tissues depends on the form in which the metal is bound (Mariam et al., 2004). Other factors that influence the accumulation of heavy metals are metal concentration in the soil, soil pH, and the physiological characteristics of the species which include assimilation and excretion capacity (Purchart and Kula, 2007). The giant African land snails are omnivores that feed on the debris from the soil surface which may have been contaminated with heavy metals and organic pollutants; therefore, they may accumulate the pollutants to harmful levels.
In A. marginata from Ikire, Cu was highest in the shell (1.030 ± 0.0071 µg/g), Ni was predominant in the digestive tract (1.199 ± 0.000 µg/g), the foot (1.107 ± 0.0014 µg/g) and the digestive gland (1.867 ± 0.208 µg/g) accumulated highest level of Cd. The results recorded in this study were within the tolerable limit of FAO/WHO (expect Ni in the digestive tract). Ogidi et al. (2020) reported (0.14 ± 0.001 µg/g), (0.032 ± 0.002 µg/g) and (0.96 ± 0.007 µg/g) for Cd, Ni and Cu, respectively in the tissue of A. marginata from Ekowe community and observed that A. marginata bioaccumulate high levels of Zinc when compared with other metals such as Cu, Cd, Ni, Cr. Iwegbue et al. (2009) recorded higher levels of Pb (6.53 ± 1.03 µg/g), Fe (7.86 ± 0.36 µg/g), Ni (0.18 ± 0.16 µg/g) and Cd (1.47 ± 0.55 µg/g) in the tissues of A. marginata in industrial sites of Warri.
The main sources of heavy metal contamination are vehicle exhaust and untreated industrial wastes that find their way through irrigation channels, therefore polluting the soil layers (Mariam et al., 2004). An increased Pb content may be found in crops and animals at distances of 50 m radius from highways, depending on weather conditions and traffic volume (Eltier and Sivacioglu, 2021). The level of heavy metals in the tissues of A. marginata from Ore is relatively higher than in A. marginata from Ikire (except Zn and Cd). Cadmium is closely related to Zinc and is found wherever Zinc is found in nature. Cd may occur as a contaminant in phosphate fertilizers and municipal sludges and so enter the food supply. Shell (2.161 ± 0.0000 µg/g) and digestive tract (2.000 ± 0.0007 µg/g) in A. marginata from Ore have a higher accumulation of Nickel. The foot accumulated Cd (2.096 ± 0.0056 µg/g) than other tissues while the digestive gland has a rich deposit of Cu (2.010 ± 0.0014 µg/g). Although, Cr and Co (in the digestive tract and gland), Ni (in the shell and digestive gland), Pb (in the digestive gland) and As (in the shell) outstripped the FAO/WHO permissible limit yet this finding is comparatively low to studies recorded by other authors. Moreover, heavy metal concentration in the muscular foot (the main constituent of snail meat) did not exceed the FAO/WHO regulated limit. Therefore, A. marginata from Ore may be tenable for human consumption. Awharitoma et al. (2016) reported higher values for Fe, Pb, Cd and Co in the range between (38.61 - 70.49 µg/g), (0.39 - 0.71 µg/g), (0.19 - 0.35 µg/g) and (0.04 - 0.007 µg/g), respectively in infected A. marginata from three communities in Edo State while Oguh et al. (2019b) reported that the concentration of heavy metals (As, Cd, Cr, Cu and Pb) in snails treated with dumpsite soil were 3.05, 3.89, 3.60, 2.89 and 2.55 mg/kg, and snails treated with mining site soil recorded 2.73, 2.74, 3.91, 4.96 and 4.82 mg/kg, respectively.
Lead (Pb) has no beneficial biological function and is known to accumulate in the body (Assi et al., 2016). Ingestion of Pb through the consumption of contaminated foods may cause mental retardation among children, inhibit haemoglobin synthesis; distort the cardiovascular system and hypertension in humans (Bello et al., 2015; Nkpaa et al., 2016). Cadmium is a toxic element because it can be absorbed through the alimentary tract and damage membrane and DNA (Maobe et al., 2012). In comparison with levels of heavy metals recorded in A. achatina by previous authors, the mean concentration of heavy metals in A. achatina collected from Ikire is low. Zn was predominant in the shell (2.003 ± 0.0014 µg/g), Cd recorded (2.306 ± 0.106 µg/g) in the foot, Ni (1.123 ± 0.0064 µg/g) was more accumulated in the digestive tract while Cd (1.227 ± 0.0063 µg/g) and Zn (1.111 ± 0.0014 µg/g) had higher accumulation in the digestive gland. Ugbaja et al. (2020) reported 1.80 µg/g of Cd in the foot of A. achatina collected from the Papalanto cement factory area. Eneji et al. (2016) recorded (0.42 - 2.80 mg/kg) of Cd in A. achatina from Abak, Akwa Ibom, Nigeria. However, Adedeji et al. (2011) had earlier recorded a low concentration (0.01 mg/kg) of Cd in snails from Alaro River in Oluyole Industrial Area Ibadan, Oyo State.
The levels of heavy metals across the tissues in A. achatina and A. marginata from Ikire and Ore were relatively within the FAO/WHO (2016) benchmarks limits except for Pb, Ni, Co and Cd in A. achatina; Pb in A. marginata from Ikire, and Cd in A. marginata from Ore which are slightly above the permissible level. This shows that the environment is gradually being polluted with toxic waste. It is important to always determine the bioaccumulation capacity for heavy metals by organisms especially the edible ones, to assess the potential risk to human health and other animals that feed on the organisms. Though, the level of heavy metals in A. marginata from Ore was comparatively higher which could probably be due to overdose of agrochemical application, higher traffic emission and higher concentration of toxic wastes from the activities of industrial presence in the Ore axis. Heavy metals often find their way into the soil and vegetation through an overdose of agrochemical application, pollution from traffic emissions and sewage from industrial estates (Adedeji et al., 2011; Eltier and Sivacioglu, 2021; Salih et al., 2021). The ability of snails to bioaccumulate essential heavy metals enables them to acquire other non-essential heavy metals from the soil and vegetation.
CONCLUSION
This study has shown that giant African land snails A. achatina and A. marginata can accumulate high levels of heavy metals in the shell and soft tissues. Thus, A. achatina and A. marginata serve as good bioindicators of heavy metal pollution in the terrestrial ecosystem. The results of this study provided baseline data on the levels of heavy metals in A. achatina in Ikire and A. marginata in Ikire and Ore. Very close monitoring of heavy metal levels in Ore and Ikire towns is recommended. Snails need to be thoroughly screened to make sure that unnecessarily high levels of toxic heavy metals are not being transferred through them to the human population that depends on the snail meat for their protein requirements. Therefore, proper monitoring of agrochemical application is recommended to reduce the level of heavy metals built-up which will contribute to further environmental pollution in the not-too-distant future.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Adedeji OB, Adeyemo OK, Oyedele MO (2011). Heavy metals in snail and water samples from Alaro River in Oluyole industrial area of Ibadan southwestern Nigeria. Journal of Applied Sciences in Environmental Sanitation 6(2):115-121. |
|
|
Ademolu KO, Idowu AB, Mafiana CF, Osinowo OA (2004). Performance, proximate and mineral analyses of African giant land snail (Archachatina marginata) fed different nitrogen sources. African Journal of Biotechnology 3(8):412-417. |
|
|
Ademuyiwa O, Agarwal R, Chandra R (2010). Effects of sub-chronic low-level lead exposure on the homeostasis of copper and zinc in rat tissues. Journal of Trace Elements in Medicine and Biology 24(3):207-211. |
|
|
Adeniyi B, Shobanke IA, Omotoso AB (2013). Economic analysis of snail meat consumption in Ibarapa Central Local Government Area of Oyo State. Journal of Marketing and Consumer Research 2:16-21. |
|
|
Afolabi OK, Wusu AD, Ogunrinola OO (2015). Arsenic-induced dyslipidaemia in male albino rats: comparison between trivalent and pentavalent inorganic arsenic in drinking water. BMC Pharmacology and Toxicology 16(1):1-15. |
|
|
Ajayi AA, Babatunde LA (2022). Observations on the digestive enzymes in the giant African land snails (Archachatina marginata): significance and prospects. Journal of Biological Sciences 22(2):50-56. |
|
|
Amobi MI, Ezewudo BI (2019). Utilization of common leafy vegetables in the diets of giant West African snail Archachatina marginata (Stylommatophora: Achatinidae). Brazilian Journal of Biological Sciences 6(12):181-187. |
|
|
Anthony AA, Adebayo-Tayo CB, Inyang UC, Aiyegoro AO, Komolafe OA (2010). Snails as meat source: epidemiological and nutritional perspectives. Journal of Microbiology and Antimicrobials 2(1):001-005. |
|
|
Appenroth KJ (2010). What are "heavy metals" in plant sciences? Acta Physiologiae Plantarum 32(4):615-619. |
|
|
Assi MA, Hezmee MN, Haron AW, Sabri MY, Rajion MA (2016). The detrimental effects of lead on human and animal health. Veterinary World 9(6):660-671. |
|
|
Awharitoma AO, Ewere EE, Alari EO, Idowu DO, Osowe KA (2016). Assessment of heavy metals in African giant snail (Archachatina marginata) and its parasites collected from three communities in Edo State, Nigeria. International Journal of Scientific and Engineering Research 6(7):1-8. |
|
|
Awodiran MO, Awopetu JI, Olayemi AO (2012). Morphometric studies of land snails, Archachatina marginata (Swainson, 1821) in some South-west, South-south and North-central states of Nigeria. International Journal of Academic Research 4(6):280-286. |
|
|
Banaee M, Taheri S (2019). Metal bioaccumulation, oxidative stress, and biochemical alterations in the freshwater snail (Galba truncatula) exposed to municipal sewage. Journal of Advances in Environmental Health Research 7(1):8-17. |
|
|
Barker GM (2001). The biology of terrestrial molluscs. CAB International, Hamilton, New Zealand P 527. |
|
|
Bello O, Naidu R, Rahman MM, Liu Y, Dong Z (2015). Lead concentration in the blood of the general population living near a lead-zinc mine site, Nigeria: exposure pathways. Science of the Total Environment 542(Pt A):908-914. |
|
|
Cowie RH, Dillon RT, Robimson DG, Smith JW (2009). Alien non-marine snails and slugs of priority quarantine importance in the United States: a preliminary risk assessment. American Malacological Bulletin 27:113-132. |
|
|
Dar MA, Pawar KD, Pandit RS (2017). Gut microbiome analysis of snails: a biotechnological approach. Organismal and Molecular Malacology pp. 189-217. |
|
|
Eltier LA, Sivacioglu A (2021). Determination of heavy metal accumulation in some coniferous species used in Kastamonu urban afforestation. Asian Journal of Biological Sciences 14:33-40. |
|
|
Eneji IS, Wuana RA, Akpan UJ (2016). Trace metals levels in African giant land snails (Achatina achatina) from selected local government areas in Akwa Ibom State, Nigeria. Open Access Library Journal 3:e2244. |
|
|
FAO/WHO (2016). List of maximum levels for contaminants and toxins in foods. Joint FAO/WHO Food Standard Programme Codex Alimentarius Commission 10th Session 150 p. |
|
|
Gawad SA (2018). Acute toxicity of some heavy metals to the freshwater snail, Theodoxus niloticus (Reeve, 1856). The Egyptian Journal of Aquatic Research 44(2):83-87. |
|
|
Gupta SK, Singh J (2011). Evaluation of molluscs as sensitive indicator of heavy metal pollution in aquatic system: a review. Environmental Management and Sustainable Development 2(1):49-57. |
|
|
Hina B, Rizwani G, Naseem S (2011). Determination of toxic metals in some herbal drugs through atomic absorption spectroscopy. Pakistan Journal of Pharmaceutical Sciences 24(3):353-358. |
|
|
Iweala EE, Olugbuyiro JA, Durodola BM (2014). Metal contamination of foods and drinks consumed in Ota, Nigeria. Research Journal of Environmental Toxicology 8(2):92-97. |
|
|
Iwegbue CM, Arimoro FO, Nwajei GE, Eguavoen O (2009). Heavy metal content in the African giant snail Archachatina marginata (Swainson, 1821) (Gastropoda: Pulmonata: Achatinidae) in southern Nigeria. Folia Malacologica 16(1):31-34. |
|
|
Izah SC, Inyang IR, Angaye TC (2017). A review of heavy metal concentration and potential health implications of beverages consumed in Nigeria. Toxics 5(1):1-6. |
|
|
Jin X, Xu Z, Zhao X (2017). The antagonistic effect of selenium on lead-induced apoptosis via mitochondrial dynamics pathway in the chicken kidney. Chemosphere 180:259-266. |
|
|
Khan FE, Jolly YN, Rabiul-Islam GM, Akhter S, Kabir J (2014). Contamination status and health risk assessment of trace elements in foodstuffs collected from the Buriganga River embankments, Dhaka, Bangladesh. International Journal of Food Contamination 1(1):1-8. |
|
|
Lemire JA, Harrison JJ, Turner RJ (2013). Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nature Reviews Microbiology 11(6):371. |
|
|
Louzon M, Gimbert F, Belly T, Amiot C, Pauget B, Vaufleury A, Capelli N (2021). From environmental bioavailability of metal(loid)s to their ecogenotoxicological effects in land snails. Environmental Science and Pollution Research 28(B):43629-43642. |
|
|
Low P, Kinga M, Gyoergy K (2016). Atlas of Animal Anatomy and Histology. Springer International Publishing AG. 413 p. |
|
|
Mahmoud KM, Abu-Taleb HM (2013). Freshwater snails as bioindicator for some heavy metals in the aquatic environment. African Journal of Ecology 51(2):193-198. |
|
|
Mani MS, Kunnathully V, Rao C (2018). Modifying effects of amino levulinate dehydratase polymorphism on blood lead levels and ALAD activity. Toxicology Letters 295:351-356. |
|
|
Maobe MAG, Gatebe E, Gitu L, Rotish H (2012). Profile of heavy metals in selected medicinal plants used for the treatment of diabetes, malaria and pneumonia in Kisii region, Southwest Kenya. Global Pharmacology 6(3):245-251. |
|
|
Mariam I, Iqbal S, Nagra SA (2004). Distribution of some trace and macrominerals in beef, mutton and poultry. International Journal of Agriculture and Biology 6:816-820. |
|
|
Nica DV, Bura M, Gergen I, Harmanescu M, Bordean D (2012). Bioaccumulative and conchological assessment of heavy metal transfer in a soil-plant-snail food chain. Chemistry Central Journal 6:55. |
|
|
Nkpaa KW, Patrick-Iwuanyanwu KC, Wegwu MO, Essien EB (2016). Health risk assessment of hazardous metals for population via consumption of seafood from Ogoniland, Rivers State, Nigeria: a case study of Kaa, B-Dere, and Bodo City. Environmental Monitoring and Assessment 188:9. |
|
|
Ogidi OI, Charles EE, Onimisi AM, Amugeh R (2020). Assessment of nutritional properties and heavy metal composition of African giant land snails (Archachatina marginata) and clams (Mercenaria mercenaria) from Ekowe community. European Journal of Nutrition and Food Safety 12(6):99-108. |
|
|
Oguh CE, Ugwu CV, Uzoefuna CC, Usman SN, Amanabo M (2019). Toxicity impact on bioaccumulation of potentially toxic elements in African giant land snail (Archachatina marginata) treated with different soils and its ecological risk assessment. Asian Journal of Research in Biochemistry 4(4):1-15. |
|
|
Oguh CE, Joseph PS, Osuji CA, Ubani CS, Okunowo WO (2019). Risk effect of water treatment sludge on bioaccumulation of heavy metals in water, fish (Oreochromis niloticus, and Clarias gariepinus) from River Chanchaga Minna Niger State, Nigeria. International Journal of Agriculture, Environment and Bioresearch 4(5):67-86. |
|
|
Okunola OJ, Alhassan Y, Yapbella GG, Uzairu A, Tsafe AI, Abechi ES, Apene E (2011). Risk assessment of using mobile phone recharge cards in Nigeria. Journal of Environmental Chemistry and Ecotoxicology 3(4):80-85. |
|
|
Purchart L, Kula L (2007). Content of heavy metals in bodies of field ground beetle (Coleoptera: Carabidae) with respect to selected ecological factors. Polish Journal of Ecology 35:305-314. |
|
|
Salih AH, Hama AA, Hawrami KA, Ditta A (2021). The land snail, Eobania vermiculata, as a bioindicator of the heavy metal pollution in the urban areas of Sulaimani, Iraq. Sustainability 13(24):13719. |
|
|
Sarah R, Tabassum B, Idrees N (2019). Bioaccumulation of heavy metals in Channa punctatus (Bloch) in river Ramganga, India. Saudi Journal of Biological Science 26(5):979-984. |
|
|
Singh S, Zacharias M, Kalpana S, Mishr S (2012). Heavy metals accumulation and distribution pattern in different vegetable crops. Journal of Environmental Chemistry and Ecotoxicology 4(10):170-177. |
|
|
Ugbaja RN, Enilolobo MA, James AS, Akinhanmi T (2020). Bioaccumulation of heavy metals, lipid profiles and antioxidant status of snails (Archachatina marginata) around cement factory vicinities. Toxicology and Industrial Health 36(20):724-736. |
|
|
Yang J, Liu D, Jing W (2013). Effects of cadmium on lipid storage and metabolism in the freshwater crab Sinopotamon henanense. PLoS One 8(10):e77569. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0