ABSTRACT
The aim of this study was to assess the microbial quality of raw beef of butcher shops around Addis Ababa city. A total of 60 samples (N=60) of raw beef were randomly collected from Dukem (N=20), Kara (N-20) and Burayu (N=20) and biologically tested for total aerobic mesophilic bacteria, total coliform, yeast and mold, Staphylococcus species, Bacillus species and psychrophilic bacteria count using standard procedures. A mean of total aerobic mesophilic bacteria, yeast and mold, Staphylococcus spp., Bacillus spp. and psychrophilic bacteria count showed highly significant difference across the locations (P<0.05) except for total coliform. Overall, mean for total aerobic mesophilic bacteria, total coliform, yeast and mold, Staphylococcus spp., Bacillus spp. and psychrophilic bacteria count was 8.34, 4.69, 6.01, 5.36, 5.45, 4.26 log 10 cfu/g, respectively. Microbial quality of raw beef collected in Dukem site was the lowest compared to Kara and Burayu while Burayu site had the highest microbial count. The presence of high microbial count in this study might indicate improper meat handling and poor sanitary condition of slaughter houses, personnel, transportation and storage. Thus, to reduce the risks of food borne bacterial infections, there is a need to educate and be aware to practice good sanitation and safe meat handling techniques for butcher shops and personnel.
Key words: Bacillus, raw beef, total coliform, total aerobic mesophilic, psychrophilic, staphylococcus, yeast, mold.
Food-borne diseases remain the most significant food safety hazards worldwide associated with beef (Maripandi and Al-Salamah, 2010) and resulting from ingestion of bacteria, toxins, and cells produced by microorganisms present in food (Clarence et al., 2009). Food and Agricultural Organization (FAO) of the United Nations and the World Health Organization (WHO) state that illness due to contaminated food is perhaps the most widespread health problem and important cause of reduced economic productivity (Käferstein, 2003). In the United States, 76 million illnesses, 325,000 hospitalizations and 5,000 deaths are caused by food-borne diseases in each year (31 known pathogens cause 9.4 million illnesses, 56,000 hospitalizations, and 1300 deaths) (Mead et al., 1999; Scallan et al., 2011). World Health Organization (WHO) has reported that 50 million children under five years of age get diarrheal diseases each year, due to the contaminated water and food stuffs (Tavakoli and Razipour, 2008). It occur commonly in developing countries particularly in Africa because of prevailing poor food handling and sanitation practices, inadequate food safety regulation, weak regulatory systems, lack of financial resources to invest in safer equipment and lack of education for food-handlers (WHO, 2004). Microorganisms, chemicals, and parasites are the leading contaminants associated with food borne acute gastroenteritis, among these; microbes are the most common contributor factor (Mohammed, 2011). Monthly, Prevalence of food borne acute gastroenteritis reported in China (10.9%) (Chen et al., 2018), Italy (8.9%), France (2.6%) and Canada (9.2%).
Contaminated raw meat is one of the major sources of food borne illnesses (Bhandare et al., 2007). Meat provides suitable media for growth of spoilage and pathogenic microorganisms. Health status of butcher shop workers, cloths and knives, wooden boards, and weighing scales can act as a source of microbial contamination (Abebe et al., 2019; Ali et al., 2010). A great diversity of microbes inhabits fresh meat generally, but different types may become dominant depending on pH, composition textures, storage temperature, and transportation means of raw meat (Adu-Gyamfi et al., 2012)
The microbial quality and safety of raw meat products can be estimated by the use of indicator microorganisms, including total aerobic plate count, coliform count and
Escherichia coli count (
Kim and Yim, 2016). The microbiological contamination of meat can occur during processing and manipulation, such as skinning, evisceration, storage and distribution at slaughter houses. Fecal matter is a major source of contamination and could reach carcasses through direct deposition, as well as by indirect contact through contaminated equipment, workers, installations and air (Pal, 2007). In Ethiopia, the consumption of raw meat has associated with cultural practices and widespread raw beef consumption habit that can be a potential source for food borne illnesses. Raw meat is available in open-air local retail shops without appropriate temperature control and purchased by households and served at restaurants as raw, slightly cooked or well cooked (Siddiqui et al., 2006)
However, there is limited information on raw beef microbial quality status in the country. Hence, this study addresses microbial quality status of raw beef from three potential butcher shops; Burayu, Dukem and Kara sites which are located around Addis Ababa city.
Study design and sample collection
A cross sectional study was conducted in retail meat shop to determine bacteriological quality of raw beef between January 2017 and 2018 G.C. Three potential butcher shops were selected purposively around Addis Ababa city and a total of sixty samples (n=60) from Dukem (n=20), Kara (n=20) and Burayu (n=20) collected early morning to minimize level of contamination due to environmental temperatures. 1 kg of cut meat collected randomly from each butcher shops and collected samples were immediately transported in insulated ice containers to Holetta Dairy Research Laboratory for microbial analysis.
Meat sample preparation
A representative samples were prepared aseptically using a sterile knife to cut smaller size. Then, 25 g of beef samples were weighed and homogenized into 225 ml of 0.1% sterile buffered peptone water using a sterile blender. Samples were centrifuged for 2 min and supernatant was transferred to test tubes for microbial analysis (FDA, 2001). Collected raw beef samples were biologically tested for total aerobic mesophilic bacteria, total coliform, yeast and mold, Staphylococcus species, Bacillus species and psychrophilic bacteria count.
Microbial analysis
Total aerobic mesophilic bacteria count
A total aerobic mesophilic bacterial count was done according to FDA (2001) using plate count agar (Oxoid, CM 0325). Serial dilution was done and 1 ml of the sample from appropriate dilution was plated in duplicate using pour plate method. The plates were incubated at 35°C for 48±2 h and the result was expressed as colony forming units per g (cfu/g).
Total coliform count
A total coliform count was done according to FDA (2001) using violet red bile agar (Oxoid, CM 0107). Then, serial dilution was done and 1 ml of the sample from appropriate dilution was plated in duplicate using pour plate method. The plates were incubated at 35°C for 24 h and the result was expressed as colony forming units per g (cfu/g).
Yeast and mold count
Yeast and mold count were carried out according to (FDA, 2001) using Potato Dextrose Agar (Himedia, M096) media. A 1ppm antibiotic (Streptomycin and Chloramphenicol) was added to sterilized PDA media. Serial dilution was done and one ml of the sample from appropriate dilution was plated in duplicate using pour plate method. The plates were incubated at 25°C for 5days in dark place and the result was expressed as colony forming units per g (cfu/g).
Staphylococcus spp.
Enumeration of Staphylococcus spp. was done using Baird-Parker Agar medium according to FDA (2001). Serial dilution was done and 0.1 ml of the sample from appropriate dilution was plated in duplicate using spread method. The plates were incubated at 35°C±2 for 48 h and colonies of Staphylococcus spp. were expressed as colony forming units per gram (cfu/g).
Psychrophilic
Psychrophilic bacterial count was done according to APHA (1984) using nutrient agar medium. Serial dilution was done and 1 ml of the sample from appropriate dilution was plated in duplicate using pour plate method. The plates were incubated at 6.5°C for 5 to 7 days and the result was expressed as colony forming units per g (cfu/g).
Bacillus spp.
The enumeration of Bacillus spp. was done using MYP agar base by spreading method (FDA, 2001). Serial dilution was done and 0.1 ml of sample from appropriate dilution was taken and spread in duplicate. Then, the plates were allowed to incubate for 24 h at 30°C and colonies of Bacillus spp. were expressed as colony forming units per gram (cfu/g).
Biochemical conformation
Staphylococcus and Bacillus spp. isolates were confirmed using morphologically, physiologically and biochemical test.
Statistical analysis
Data was analyzed using Statistical Analysis Software (SAS Inc., Cary, USA, version 9) and ANOVA was applied to compare the means of study sites. Microbial count of cfu results was log transformed before the statistical analysis and the mean separation was done by using Duncan's Multiple Range Test.
Total aerobic mesophilic bacteria
The mean of total aerobic mesophilic bacteria in Dukem and Kara was 8.09 and 8.10 log10 cfu/g which was almost the same. This may indicate similarity of sanitary and hygienic practices between two locations. The study showed, there were highly significant differences (P<0.05) across the locations (Table 1). The mean count of total aerobic mesophilic bacteria at Burayu was the highest compared to Dukem and Kara samples. High level of total aerobic bacteria might indicate, the possibility of oxygen demanding microorganism on raw beef. The overall mean of total aerobic mesophilic bacteria in this study revealed 8.34 log10 cfu/g. However, HPA (2009) indicated aerobic mesophilic count must be less than 7 log cfu/g for raw meat. The present result is relatively higher than mean of fresh meat of 4.53 and 5.210 log10 cfu/g in Bahirdar and Adama town (Melkamnesh and Mulugeta, 2017; Gebeyehu, et al. (2013) and Elsharawy et al. (2018) revealed that aerobic plate count of 5.6 Log CFU/g from beef samples collected from Ismailia city abattoir, Egypt.
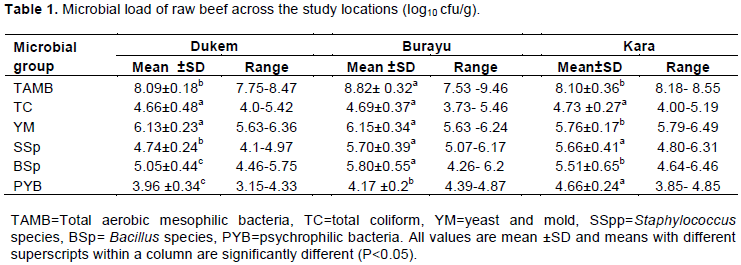
High total aerobic mesophilic bacteria count reveals potential indicators for possible presence of pathogenic microorganisms, poor sanitation and cross contamination. According to Firew et al. (2014) observational study in Jigjiga town indicated that poor sanitation and dusty environment and full remains of slaughtered animals such as bones, horn, head and other body parts observation were correlated with total aerobic mesophilic bacterial count. Hence, consumption of raw beef meat has healthy risk unless heat treating (frying) or other optimal food processing methods. Abebe et al. (2019) reported that Bisheftu butcher shops had poor hygienic condition and butcher shops workers had a low education status. Unclean slaughter houses and butcher shops, handling of meat and hot environmental condition are main source of contamination (Bhandare et al., 2007). Firew et al. (2014) revealed that, only 67% of the vendors in Jigjiga town had relatively good personal hygiene with respect to cleanness of their cloths and visible body parts.
Total coliform
The mean of total coliform count from Dukem, Burayu and Kara was 4.66, 4.69 and 4.73 log10 cfu/g, respectively. Total coliform count from three study locations ranged between 4.66 and 4.73 log10 cfu/g. This indicates microbial quality of each samples is in close proximity due to comparable hygienic condition and post handling practices. The overall mean of total coliform count in this study was 4.69 log10 cfu/g. The higher coliform counts correlate with the higher levels of food-borne pathogens of fecal origin (Jay, 2000). According to HPA (2009), raw meat is categorized as unacceptable if the count of Enterobacteriaceae and coliform is greater than 4 log10 cfu/g. But, less total coliform count was observed contrast to Francis et al. (2015) who reported the mean of total coliform count of raw beef collected from local super market in Ghana was 7.23 log10 cfu/g. However, the present result was higher than that of Firew et al. (2014) finding who reported the mean of total coliform of raw beef was 4.45 log10 cfu/g in South East Ethiopia, Jigjiga town.
Yeast and mold
Yeast and mold counts in raw beef collected from the study site had highly significant difference across the location (P<0.05). Yeast and mold counts of beef samples collected from Kara had significant difference from beef samples collected from Dukem and Burayu sites (P<0.05). The mean value of yeast and mold counts obtained from this study was 6.01 log10 cfu/g. The minimum and maximum mean value of yeast and mold in this study was 5.76 and 6.49 cfu/g, respectively. Sanjay (2019) indicated that, high load of yeast and mold has direct relation with sanitation parameters. The mean of yeast and mold was less compared to yeast and mold in raw beef. This might happen due to the difference of pH in raw beef collected from different study locations.
Staphylococcus spp.
Staphylococcus spp. count in raw beef collected from the study sites had highly significant differences (P<0.05) across the study areas (Table 1). The mean value of Staphylococcus spp. count from all study areas was 5.4 log10 cfu/g. The result of this study was relatively lower than 5.5×105 cfu/g reported by Gebeyehu et al. (2013) in Adama town. The present result of Staphylococcus spp. figure may indicate poor sanitary condition in slaughter houses, transportation and blucher shops or due to contamination from the skin, mouth, or nose of the handlers can be major cause of high prevalence. Risk factors study by Adugna et al. (2018) who reported high prevalence of Staphylococcus from swap samples collected from cutting table, knife and hooks support the study. The current study correlated with risk factors study, conducted at Addis Ababa City, in which a total of 24 butcher shops were interviewed and 75% of the workers did not wear aprons and 58.3% of them did not cover their hair; 41.1% of the butcher shop workers used only water for cleaning (Adugna et al., 2018).
Bacillus spp.
There was a significant difference in mean value of Bacillus spp. count for raw beef among the study locations (P<0.05). The present study showed the highest mean value Bacillus spp. count was found in Burayu samples (5.80 log10 cfu/g), while the lowest mean value was found in Kara (5.05 log10 cfu/g). The overall mean value of Bacillus spp. count of raw beef was 5.45 log10 cfu/g. The result obtained is higher than Bradeeba and Sivakumaar (2012) who reported the mean value of raw beef of Bacillus spp. count was 2.68 log10 cfu/g in retail shops. Detection of Bacillus spp. to this level may indicate the favorable condition for Bacillus cereus presence which is food borne pathogen which can survive in harsh environments including normal cooking temperature. Thus, the possibility of raw beef consumption can be health risk and cause either emetic or diarrheal syndrome survives. Although, it is recommended to process raw beef, there will be possibility outbreak if processed food subjected tenderization processes. This happens under slow cooling and storage large amounts of cooked foods at temperatures between 10 and 50°C favor Bacillus spp.
Psychrophilic bacteria
The mean value of psychrophilic bacteria count of raw beef samples in Dukem, Burayu and Kara was 3.96, 4.17, and 4.66 log10 cfu/g, respectively. The overall mean value of psychrophilic bacteria count in raw beef was 4.26 log10 cfu/g. The result obtained was greater than the finding by Bradeeba and Sivakumaar (2012) which found 3.56 log10 cfu/g retail shops in Tamil Nadu. High load of psychrophilic bacteria at fresh meat might indicates possibility the flora survive even under refrigerated condition. This study can be supported by the work of Bouzid et al. (2015) who revealed psychrophilic bacteria presence in frozen meat.
The results indicated that, the highest total aerobic mesophilic bacteria, yeast and mould, Staphylococcus spp. and Bacillus spp. count were recorded from Burayu samples. Whereas, Dukem had the lowest total aerobic mesophilic bacteria, total coliform, yeast and mold, Staphylococcus spp., Bacillus spp. and psychrophilic bacteria count (Figure 1).
CONCLUSION AND RECOMMENDATION
Based on the present results, microbial load of raw beef in the study locations is high which can be indicated to poor hygienic conditions in slaughter house, transportation and butcher shops. Hence, consuming raw beef has a healthy risk for consumers. Selling raw beef in open place and unhygienic environment, lack of cold storage transportation, lack of awareness on food safety and poor sanitation level of slaughtering houses can be main risk factors for high contamination level of raw beef for bacteria. Thus to reduce the risks of food borne bacteria, there is need to educate on practicing good sanitation and handling techniques guidelines, monitoring safety status and enforcing food safety rule and regulations for public health security and enhance safe meat production. Developing an appropriate Hazard Analysis Critical Control Point system will be important program to identify and control the hazards to improve meat microbial quality supplied to consumer and ensure food safety and quality.
The authors have not declared any conflict of interests.
REFERENCES
|
Abebe B, Dereje T, Chaluma N (2019). Investigation of Bacteriological Quality of Meat from Abattoir and Butcher Shops in Bishoftu, Central Ethiopia. International Journal of Microbiology Article ID 6416803:8.
Crossref
|
|
|
|
Ali N, Farooqui A, Khan A, Kazmi S (2010). Microbial contamination of raw meat and its environment in retail shops in Karachi, Pakistan. Journal of Infection in Developing Countries 4(6):382-388.
Crossref
|
|
|
|
|
Adu-Gyamfi A, Torgby-Tetteh W, Appiah V (2012). Microbiological Quality of Chicken Sold in Accra and Determination of D10-Value of E. coli. Food and Nutrition Science 3(5):693-698
Crossref
|
|
|
|
|
Adugna F, Pal M, Girmay G (2018). Prevalence and Antibiogram Assessment of Staphylococcus aureus in Beef at Municipal Abattoir and Butcher Shops in Addis Ababa, Ethiopia. BioMed Research International Article ID 5017685:7.
Crossref
|
|
|
|
|
American Public Health Association (APHA) (1984). Compendium of Methods for Microbiological Examination of Food. Speck, M.L. Ed. American public Health Association, Washington, DC.
|
|
|
|
|
Bhandare SG, Sherikarv AT, Paturkar AM, Waskar VS, Zende RJ (2007). A comparison of microbial contamination on sheep/goat carcasses in a modern Indian abattoir and traditional meat shops. Food Control 18(7):854-858.
Crossref
|
|
|
|
|
Bradeeba K, Sivakumaar PK (2012). Assessment of microbiological quality of beef, mutton and pork and its environment in retail shops in chidambaram, tamil nadu. International Journal of Plant, Animal and Environmental Science 3:1.
|
|
|
|
|
Bouzid R, Guemour D, Zidane K, Aggad H, Bendella A, Saegerman C (2015). Hygienic quality of minced meat retailed in western Algeria. Journal of Virology and Microbiology, pp. 5-14.
Crossref
|
|
|
|
|
Chen Y, Yufeng W, Jiangen S, Baifeng C, Shushu D, Lei D, Jiajia D (2018). The correlation between family food handling behaviors and food borne acute gastroenteritis: a community-oriented, population-based survey in Anhui, China. BMC Public Health 18:1290.
Crossref
|
|
|
|
|
Clarence Y, Obinna CN, Shalom NC (2009). Assessment of bacteriological quality of ready to eat food (Meat pie) in Benin City metropolis, Nigeria. African Journal of Microbiology Research 3(6):390-399.
|
|
|
|
|
Elsharawy NT, Ahmad AM, Abdelrahman HA (2018). Quality Assessment of Nutritional Value and Safety of Different Meat. Journal of Food Microbiology Safety Hygiene 3:132.
Crossref
|
|
|
|
|
Food and Drug Administration (FDA) (2001). Bacteriological Analytical Manual U.S.
|
|
|
|
|
Firew T, Gulelat D, Ketema B, Haile A (2014). Microbiological quality and safety of street vended raw meat in Jigjiga town of Somali Regional State, Southeast Ethiopia. African Journal of Microbiology Research 8(48):3867-3874.
|
|
|
|
|
Francis AH, Abraham AG, Victoria A (2015). Microbiological and parasitological quality of local beef retailed in Accra and radiation sensitivity of Salmonella sp. International Journal of Current Microbiological and Applied Sciences 4(4):86-96.
|
|
|
|
|
Gebeyehu A, Yousuf M, Sebsibe A (2013). Evaluation of Microbial Load of Beef of Arsi Cattle in Adama Town, Oromia, Ethiopia. Journal of Food Process Technology 4:234.
Crossref
|
|
|
|
|
Health Protection Agency (HPA) (2009). Guidelines for assessing the microbial safety of ready to eat foods. London: Health Protection Agency.
|
|
|
|
|
Jay JM (2000). Food-borne gastroenteritis caused by Escherichia coli. In: Jay J (Ed.), Modern Food Microbiology (5th ed., 531-543). New York, NY: Chapman and Hall.
Crossref
|
|
|
|
|
Käferstein F (2003). Food safety as a public health issue for Developing Countries. Focus 10, brief 2 of 17. 2020 Vision for Food, Agriculture and the Environment. Washington, DC., USA.
|
|
|
|
|
Kim JH, Yim DG (2016). Assessment of the microbial level for livestock products in retail meat shops implementing HACCP system. Korean Journal of Food Science 36:594-600.
Crossref
|
|
|
|
|
Maripandi A, Al-Salamah AA (2010). Multiple-antibiotic resistance and plasmid profiles of Salmonella enteritidis isolated from retail chicken meats. American Journal of Food Technology 5:260-268.
Crossref
|
|
|
|
|
Mead PS, Slutsker L, Dietz V (1999). Food-related illness and death in the United States. Emerging Infectious Diseases 5(5):607-625.
Crossref
|
|
|
|
|
Melkamnesh A, Mulugeta K (2017). The Bacteriological Quality, Safety, and Antibiogram of Salmonella Isolates from Fresh Meat in Retail Shops of Bahir Dar City, Ethiopia. International Journal of Food Science Article ID 4317202:5.
Crossref
|
|
|
|
|
Mohammed FA (2011). The incidence of enterobacteriaceae causing food poisoning in some meat products. Journal of Food Science Technology 3(2):116-21.
|
|
|
|
|
Pal M (2007). Zoonoses, 2nd edn. Satyam Publishers, Jaipur, India pp. 104-105.
|
|
|
|
|
Sanjay M (2019). Relationship of Sanitation Parameters with Microbial Diversity and Load in Raw Meat from the Outlets of the Metropolitan City Biratnagar, Nepal. International Journal of Microbiology. Article ID 3547072:17.
Crossref
|
|
|
|
|
Scallan E, Hoekstra RM, Angulo FJ (2011). Food borne illness acquired in the United States-major pathogens. Emerging infectious Diseases 17(1):7-15.
Crossref
|
|
|
|
|
Siddiqui FJ, Rabbani F, Hasan R, Nizami SQ, Bhutta ZA (2006). Typhoid fever in children: some epidemiological considerations from Karachi, Pakistan. International Journal of infectious Disease10:215-222.
Crossref
|
|
|
|
|
Tavakoli HR, Razipour M (2008). Microbial quality of cooked meat foods in Tehran Universities Restaurants, Pakistan Journal of Medical Science 24:595-599.
|
|
|
|
|
World Health Organization (WHO) (2004). Regional Office for Africa "Developing and Maintaining Food Safety Control Systems for Africa Current Status and Prospects for Change", Second FAO/WHO Global Forum of food Safety Regulators, Bangkok, Thailand pp. 12-14.
|
|