ABSTRACT
Mycosis constitutes a common health problem, especially in developing countries like Nigeria. The current cascade of antifungals are either too toxic or require long term use for total eradication. This study evaluates antifungal activity and phytochemical constituents of Luffa cylindrica leaves extract which were screened for the presence of bioactive phytochemicals and extracted by cold maceration in n-hexane, ethyl acetate, methanol and water. The ethyl acetate extract was further fractionated using bio-assay guided column chromatography. In vitro antifungal activities were investigated against three types of fungi which were, Candida albicans ATCC 2876, Candida tropicalis ATCC 19092, Trichophyton rubrum ATCC 28188; and four clinical isolates of C. albicans, C. tropicalis, Microsporum canis and Epidermophyton flocossum using agar diffusion and micro broth dilution methods. The crude extracts revealed the presence of sterols, saponins, flavonoids, phenols and alkaloids. The ethyl acetate extract produced the strongest antifungal activity with diameter zones of inhibition ranging from 13.00 to 16.00 mm at an exposure concentration of 2500 µg/mL. The minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) values of the ethyl acetate extract against the test fungi were 500 to 1000 µg/mL and 2000 to 4000 µg/mL, respectively. L5 and L6 fractions produced fungal inhibitory activity comparable to the crude ethyl acetate extracts of L. cylindrica with MIC values of 1000 to 2000 µg/mL and 500 to 2000 µg/mL, respectively. The ethyl acetate extracts of L. cylindrica possess antifungal properties that could serve as leads for the development of novel antifungal drugs.
Key words: Luffa cylindrica leaves, antifungal, phytochemical screening, minimum inhibitory concentration.
In the past few decades, there has been a worldwide increase in the incidence of fungal infections due to a rise
In the resistance of some species of fungi to current antifungal agents used in medicinal practice (Abad et al., 2007; Senguttuvan et al., 2013; Dzoyem et al., 2014). The rise in the incidence of fungal infections has exacerbated the need for the next generation of antifungal agents, since many of the currently available drugs have undesirable side effects, and are ineffective against new or re-emerging fungi, or lead to the rapid development of resistance (Kullberg and Filler, 2002; Kreander et al., 2005). There has been little break-through in the research into development of new antifungal drug unlike antibacterial agents (Scorzoni et al., 2017). Superï¬cial mycosis are the most prevalent fungal infections, with infection rate in humans worldwide of about 25%, however invasive fungal infections (systemic) are more life-threatening, difficult to diagnose with limited amount of therapeutic options and accounting for approximately 1.5 million deaths annually (Souza and Amaral, 2017).
Owing to the few antifungal arsenal, researchers have explored several approaches, with the most recent and effective development being the application of nanotechnology thus employing nanoparticles as carrier for antifungal drugs (Scorzoni et al., 2017). Luffa cylindrica (L.) M. Roem belongs to the family Curcubitaceae. It is also known as Momordica cylindrica L. (1753), Luffa aegyptica Mill. (1768). L. cylindrica is widely distributed in the tropics and subtropics, as a cultivated and naturalized plant. Its cultivation is of ancient origin and it is hard to determine whether the native home is Africa or Asia. L. cylindrica has been reported to possess both medicinal and nutritional properties (Partap et al., 2012). Its seeds have been used in the treatment of asthma, sinusitis and fever (Sashikala et al., 2009). Its use in AIDS management can be linked to the presence of proteins such as luffaculin with ribosome-inhibiting properties on the replication of HIV infected lymphocyte and phagocyte cells (Otimenyin et al., 2008). Abirami et al. (2011) reported that juice extracted from the stem is used in the treatment of respiratory disorders and the seed has emetic action. The aim of this study was to screen for phytochemical constituents and evaluate the antifungal activities of the L. cylindrica leaf extracts.
Chemicals and media
Dimethyl sulphoxide (DMSO), fluconazole (Cat No. F8929), terbinafine HCl (T8826), n-hexane, ethyl acetate, and ethanol were obtained from Sigma Aldrich Laboratories, Germany. Sabouraud dextrose agar (SDA) and Sabouraud dextrose broth (SDB) were obtained from Oxoid, Germany.
Plant collection
The fresh leaves of L. cylindrica were collected from the botanical garden of the National Institute for Pharmaceutical Research and Development (NIPRD), Abuja, Nigeria between September and October, 2014. Voucher specimen (NIPRD/H/6643) of plant was deposited in the herbarium at the Department of Medicinal Plant Research and Traditional Medicine, NIPRD, Abuja Nigeria. The fresh leaves were separated, shade dried and grinded into powder using mortar and pestle.
Test organisms
Candida albicans ATCC 2876, Candida tropicalis ATCC 19092, clinical strains of C. albicans, C. tropicalis, Trichophyton rubrum ATCC 28188, clinical strains of Microsporum canis and Epidermophyton floccosum were obtained from Department of Microbiology and Biotechnology, NIPRD, Abuja, Nigeria. Suspensions of fungi were made in SDB. Subsequent dilutions were prepared from the above suspensions and used in the tests.
Extract preparation
Five hundred grams (500 g) each of the powdered leaves was macerated in various solvents (n-Hexane, ethyl acetate, methanol and water) with random shaking for 72 h and filtered. After each extraction, the extracts were concentrated using a rotary evaporator and water bath, dried and weighed.
Phytochemical screening
The freshly prepared extracts of the powdered L. cylindrica were evaluated for the presence of carbohydrate, tannins, flavonoids, phlobatannins, saponins, alkaloids, terpenes, sterols, phenols, resins and anthraquinone using simple qualitative and quantitative methods of Sofowora (1993) and Evans (2004).
Fractionation of ethyl acetate extract of L. cylindrical
Column chromatography was used to further simplify the solvent extract with highest antifungal activity from previous extraction (Masoko and Eloff, 2005). The wet method for packing of chromatographic columns was used; silica gel 60 was made into slurry with the least polar solvent and then poured slowly into a column (40.5 cm

3.0 cm), on top of a small amount of cotton wool. The sample was dissolved in small quantity of appropriate solvent and then triturated thoroughly with equal weight of silica gel 60 in a mortar and pestle and the mixture was allowed to air dry. The extract- silica gel mixture was made into slurry with the most non polar solvent and poured neatly on top of the silica in the column.
Filter paper cut to the internal diameter of the column and cotton-wool was neatly placed on top of the sample to prevent disturbance at the surface during solvent introduction. The appropriate elution systems were added slowly in the increasing order of their polarity. The fractions were eluted with n-hexane (100%), n-hexane – ethyl acetate (90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 20:80, 10:90), ethyl acetate (100%), ethyl acetate – methanol (90:10, 80:20, 60:40, 40:60), methanol (100%), methanol – water (90:10, 80:20, 60:40). With the addition of solvent into the column, the vacuum was switched on. The solvent was allowed to run through the column; until all the solvent had been collected in the beakers through a separating funnel. The solvents collected in the beakers were concentrated using a rotary evaporator and TLC analysis carried out.
Preparation/ standardization of fungi
The yeast (Candida sp.) was standardized by inoculating sterile normal saline solution with a 48 h pure culture by adjustment of turbidity to match 0.5 Mc Farland standards. Standardization of the dermatophytes included harvesting fungal spores from a 7 day old culture on SDA slant. Ten milliliters (10 mL) of sterile normal saline containing 3% (w/v) Tween 80 was used to disperse the spores with the aid of sterilized glass beads (Olowosulu et al., 2005). Standardization of the spore suspension to (1.0 x 106 spores/mL) was achieved with a UV spectrophotometer (Spectronic 20D; Milton Roy Company, Pacisa, Madrid, Spain) at 530 nm (OD530) of the suspensions and adjusted to a transmittance of 70 to 72%. The standardized fungal suspensions were quantified by spreading 100 µL on Sabouraud dextrose agar plate. The plates were incubated 24 h at 37°C for yeast and 72 h at 30°C for dermatophytes (Aberkane et al., 2002).
In vitro assessment of antifungal activity
Cup plate agar diffusion method (Etuk et al., 2008) was used to assess the antifungal activity of the extracts. Eighteen hours culture of Candida spp. and inoculum suspensions of the dermatophytes prepared from fresh, mature (7 to 14 day old) cultures in Sabouraud dextrose liquid medium were standardized to produce inoculum size of 106 cfu/mL. One millilitre (1 mL) of the diluted culture of each test organism was used to flood Sabouraud dextrose agar media and excess aseptically drained. The plates were allowed to dry at 37°C in a sterilized incubator. Adopting the agar diffusion cup plate method (Olowosulu et al., 2005), a sterile cork borer (6 mm) was used to bore holes in the agar plates. The bottoms of the wells (holes) were sealed with the appropriate molten Sabouraud dextrose agar. Using micropipette, 0.1 ml each of the different graded concentrations of the ethyl acetate extract was dispensed into the holes marked 'A' (20 mg/mL), 'B' (10 mg/mL), 'C' (5 mg/mL) and 'D' (2.50 mg/mL). Distilled water and the solvents used in diluting the extracts were used as control. These were allowed to diffuse into the agar at room temperature for 1 h before incubation at 37°C for 18 h (yeast) and 30°C for 72 h (dermatophytes). The zones of inhibition of the test organisms were measured to the nearest millimetre, using a well-calibrated meter ruler and pair of dividers. The experiment was carried out in triplicates (Olowosulu et al., 2005).
Determination of Minimum Inhibitory Concentration (MIC)
The MIC value of the extracts and fractions against the fungal strains was determined using broth microdilution bioassay with tetrazolium violet reduction as an indicator of growth (Pereira et al., 2011). The 96-well plates were prepared by dispensing 50 μL of Sabouraud dextrose broth into each well and 50 μL from the stock suspension of plant extracts and fractions was added into the first wells. Then, 50 μL from their serial dilutions was transferred into consecutives wells, excluding the last ones. The last well contained 50 μL of broth inoculated with fungal inoculum to confirm the cell viability (viability control). At the same way positive controls were carried out with standard antifungal using terbinafine HCl and fluconazole. Sterility control was performed to verify whether the broth used in antifungal assay was contaminated before test procedures. For that, 50 μL of broth was dispensed into a well, without both extract and inoculum. As an indicator of growth, 40 μL of 0.2 mg/mL of p-iodonitrotetrazolium violet was added to each of the microplate wells.
Determination of the Minimum Fungicidal Concentration (MFC)
Minimum fungicidal concentration was determined using the micro dilution method to verify if the inhibition was reversible or permanent. Aliquot of 50 μL from the wells that did not show growth in MIC procedure (inactivated with 10% tween 80) was transferred to 96-well plates previously prepared with 50 μL of SDB. The plates were aseptically sealed followed by mixing on plate shaker (300 rpm) for 30 s, incubated at 30°C for 2 to 7 days. The test was performed in triplicate and the geometric mean values were calculated. Minimum fungicidal concentration was defined as the lowest extract concentration in which no visible growth occurred when sub cultured on the 96-well plates containing broth without antifungal products.
Statistical analysis
Data obtained were expressed as mean ± standard deviation and analyzed for significance using Students t-test and one way ANOVA (GraphPad Prism 5) at p < 0.05.
The yield of the different solvent extracts as shown in Table 1 revealed an increase in the extraction yield with increase in the polarity of the extraction solvents. As a result, water (most polar) yielded the greatest quantities and less polar solvent n-hexane extracted the least amount. The yield of the hexane, ethyl acetate, methanol and water were 2.0, 3.65, 4.0 and 4.56% respectively. The phytochemical screening of the extracts L. cylindrica revealed the presence of carbohydrates, sterols, saponins, flavonoids, alkaloid and phenols; while resins, tannins, terpenes, balsams and anthraquinones were not detected. Phytochemical screening of Fractions F5 and F6 revealed the absence of flavonoids and phenols (Table 2). The result on the susceptibility of the various extracts at a concentration of 20 mg/mL on the growth of the test fungi is represented in Table 3.
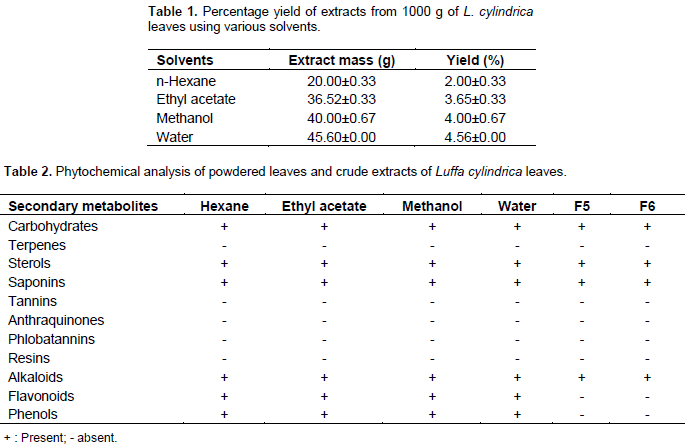
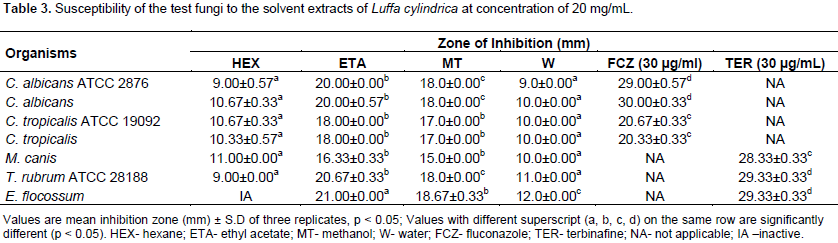
Generally, all the extracts showed a level of inhibition against all the fungi tested with exception of n-hexane extracts which exhibited no inhibitory activity against E. flocossum. However, the ethyl acetate and methanol extract produced the strongest antifungal activities which were comparable with the standard antifungals used. The strongest inhibitory activity was exhibited by the ethyl acetate extracts with a diameter zone of inhibition range of 18.0 to 21.0 mm, this was followed by methanol extracts (15.0 to 18.7 mm) and least by the water and n-hexane extracts with zones of inhibition range of 9.0 to 12.0 and 9.0 to 11.0 mm, respectively. Result of the effects of increasing concentration of the crude ethyl acetate and methanol extracts of L. cylindrical are shown in Tables 4 and 5, respectively.
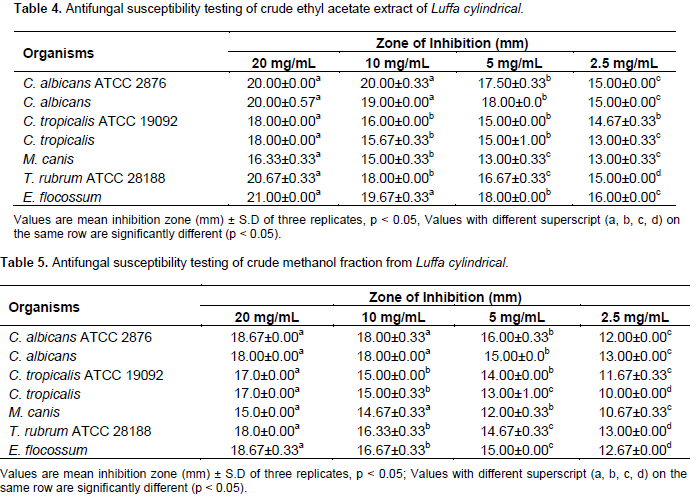
The results revealed a gradual increase in the inhibitory action of the extracts with increase in concentration of the extracts. However the ethyl acetate extracts (13.0 to 20.0 mm) showed stronger antifungal activity than the methanol extract (10.0 to 18.0 mm) against all the fungi tested. Fractions L5 and L6 (ethyl acetate extract) produced fungal inhibitory activity comparable to the crude ethyl acetate extracts of L. cylindrica with MIC values of 1000.0 to 2000.0 µg/mL and 500.0 to 2000.0 µg/mL, respectively, and MFC values range of 4000.0 to 8000.0 µg/mL and 2000.0 to 4000.0 µg/mL, respectively. The MIC of fraction L5 though comparable with the crude extract, however is not as active against T. rubrum (1000.0 µg/ml) while L6 was most active against C. albicans (500.0 µg/mL) with MIC similar to the crude extract.
The biological activities of the extract have also been linked to the solvent polarity (Zohra and Fawzia, 2011). Ahmad et al., (2009) reported that the yield of extraction is greatly influenced by the polarities of solvents; the more polar solvent produces higher yields than the less polar solvents. The extraction yield of the aqueous extract was lower than those previously reported by Mhya et al. (2014), who reported a percentage yield of 17.75. The lower yield recorded in this study could be linked to differences in the season of collection and location of the plant. Plants possess bioactive phyto-compounds like saponins, tannins, flavonoids, and alkaloids etc. which have been shown to be responsible for their antimicrobial potentials (Thamaraiselvi and Jayanthi, 2012). The presence of carbohydrates, sterols, saponins, alkaloids, flavonoids and phenols in the study plant agrees with studies reported in the past (Aboh et al., 2012; Sharma, 2012; Mhya et al., 2014). However, the absence of glycosides in the study contrasts previous reports (Sharma, 2012; Mhya et al., 2014), tannins (Mhya et al., 2014).
This could be related to the period of collection, methods of extraction or location of the plants. The botanical source and location of plants have been shown to affect or alter the presence and abundance of phytochemicals present in a plant, which can in turn affect its biological properties (Aliero and Wara, 2009; Prashant et al., 2011). Solvents employed in extraction of phyto-compounds from medicinal plants play a vital role in the degree of biological activities the plant will exhibit. The phyto-constituents present in plants possess varying degree of solubility in different solvents, which is due to the different classes of constituents present in the plant and the polarities of the solvents (Olowosulu et al., 2005). The strong growth inhibition and broad spectrum (yeast and dermatophytes) of activity displayed by the ethyl acetate and methanol extract as compared to the water and hexane extracts could be linked to the ability of these solvents to extract more anti-fungal components of the plants than the other solvents.
The large growth inhibition (17.0 to 18.67 mm and 18.0 to 21.0 mm) of Candida sp by the methanol and ethyl acetate extracts of L. cylindrica which was found to be comparable with the standard antifungals is in agreement with previous studies (Aboh et al., 2012; Ahmad and Khan, 2013; Aladejimokun et al., 2014). The crude extracts however showed the least anti-fungal activity against M. canis with zone of inhibition range between 10.0 and 16.33 mm at a concentration of 20 mg/mL. Ahmad and Khan (2013), reported linear growth inhibition of 70 and 75% against M. canis by the ethyl acetate fraction and crude methanolic extract, respectively, of L. cylindrica at a concentration of 24 mg/mL. Generally, the ethyl acetate fraction produced strongest antifungal activities against the fungi tested. This could be linked to the ability of ethyl acetate to solubilize and extract the antifungal components of the plant. Aboh et al., 2014, reported that non polar solvents like ethyl acetate and acetone are able to extract antifungal constituents from medicinal plants than polar solvents.
The increasing antifungal activities of the crude ethyl acetate extract with increase in concentration, is an indication of the potency of the extracts. However the significance of the increment varied among the test organism, as in most cases the difference in activity at concentrations of 5mg/mL and 10 mg/mL was not significant. The antifungal activities of the crude methanolic extracts also followed a similar pattern, however with lower antifungal inhibitory potentials. This agrees with the work by Ahmad and Khan (2013), which recorded a stronger antifungal (dermatophytes) activity by the ethyl acetate extract of L. cylindrica over the methanolic extract. The reduced antifungal activities of fractions (F5 and F6) as compared to the crude ethyl acetate extract are not surprising. Also the absence of flavonoids and phenols could be associated with their reduced antifungal action. . Previous study has reported a reduced biological action of the fractions of some medicinal plants as compared to the crude plants (Hefferon, 2012). This suggests that the biological activity of some plant is due to a combination of phytochemicals which are separated into smaller entities with varying biological efficacy during the process of fractionation (Shafi et al., 2013). These lower antifungal activities of the fractions to the crude as explained suggest loss of active components during fractionation process.
The extracts and fractions L5 and L6 of the leaves of L. cylindrica produced good antifungal activity against a broad class of fungi. The ethyl acetate extract was shown to be most effective and could serve as a lead for development of novel antifungals in the nearest future.
The authors have not declared any conflict of interests.
The authors are grateful to the Management of the National Institute of Pharmaceutical Research and Development for provision of an enabling environment for the study. the contributions of Mallam Mu’azam, Mr John Apev, Mr Andrew Sule and Mr. Ibrahim Ijele in the collection and screening of the plant are also appreciated.
REFERENCES
|
Abad MJ, Ansuategui M, Bermejo P (2007). Active antifungal substances from natural sources. Arch. Org. Chem. 7:116-145.
|
|
|
|
Aberkane A, Cuenca-Estrella M, Gomez-Lopez A, Petrikkou E, Mellado E, Monzon A, Rodriguez-Tudela J, Eurofung LN (2002). Comparative evaluation of two different methods of inoculum preparation for antifungal susceptibility testing of filamentous fungi. J. Antimicrob. Chem. 50:19-22.
Crossref
|
|
|
|
|
Abirami SM, Indhumathy R, Sashikala DG, Kumar DS, Sudarvoli M, Nandini R (2011). Evaluation of the wound healing and anti-inflammatory activity of whole plant of Luffa cylindrica (Linn). in rats. Pharmacol. Online 3:281-285.
|
|
|
|
|
Aboh MI, Okhale SE, Ibrahim K (2012). Preliminary studies on Luffa cylindrica: Comparative phytochemical and antimicrobial screening of the fresh and dried aerial parts. Afr. J. Microbiol. Res. 6(13):3088-3091.
|
|
|
|
|
Aboh MI, Olayinka BO, Adeshina GO, Oladosu P (2014). Antifungal activities of phyto compounds from Mitracarpus villosus (Sw.) DC aerial parts obtained from Abuja, Nigeria. Malays. J. Micro. 10(2):133-138.
|
|
|
|
|
Ahmad A, Alkarkhi A, Hena S, Khim LH (2009). Extraction, separation and identification of chemical ingredients of Elephantopus scaber L. using factorial design of experiment. Int. J. Chem. 1(1):36- 49.
Crossref
|
|
|
|
|
Ahmad B, Khan AA (2013). Antibacterial, antifungal and phytotoxic activities of Luffa cylindrica and Momordica charantia. J. Med. Plants Res. 7(22):1593-159
|
|
|
|
|
Aladejimokun AO, Adesina IA, Falusi VO, Edagbo DE (2014). Comparative study of antimicrobial potency and phytochemical analysis of methanolic extracts of the leaf and flower of Luffa cylindrica. J. Nat. Sci. Res. 4(8):7-10.
|
|
|
|
|
Aliero AA, Wara SH (2009). Validating the medicinal potential of Leptadenia hastata. Afr. J. Pharm. Pharmacol. 3(6):335-338.
|
|
|
|
|
Dzoyem JP, Tchuenguem RT, Kuiate JR, Teke GN, Kech, FA, Kuete V (2014). In vitro and in vivo antifungal activities of selected Cameroonian dietary spices. BMC Complem. Altern. Med. 14(58):1-8.
Crossref
|
|
|
|
|
Etuk EU, Suberu, HA, Ameh, IG, Abubakar K (2008). Antimycotic effect of the aqueous leaf extract of Pterocarpus erinaceus in rats. J. Pharm. Toxic. 3:318-323.
Crossref
|
|
|
|
|
Evans WC (2004). Trease and Evans Pharmacognosy. WB Saunders Ltd. London, pp. 32-33, 95-99, 512, 547.
|
|
|
|
|
Hefferon KL (2012). Plant-made vaccines. J. Vac. Vaccin. 3(4):108.
Crossref
|
|
|
|
|
Kreander B, Vuorela KP, Tammela P (2002). A rapid screening method for detecting active compounds against erythromycin-resistant bacterial strains of Finnish origin. Fol. Microbiol. 50(6):487-493.
Crossref
|
|
|
|
|
Kullberg B, Filler S (2002). Candidemia: In Candida and Candidiasis. ASM Press, Washington DC. 2002:327-340.
|
|
|
|
|
Masoko P, Eloff JN (2005). The diversity of antifungal compounds of six South African Terminalia species (Combretaceae) determined by bioautography. Afr. J. Biotechnol. 4(12):1425-1431.
|
|
|
|
|
Mhya DH, Mankilik M (2014). Phytochemical screening of aqueous extract of Luffa aegyptiaca (sponge gourd) leave sample from Northern Nigeria: A short communication. Int. J. Pharmaceut. Sci. Res. 5(7):344-345.
|
|
|
|
|
Olowosulu AK, Ibrahim YKE, Bhatia PG (2005). Studies on the antimicrobial properties of formulated creams and ointments containing Baphia nitida heartwood extract. J. Pharm. Biores. 2(2):124-130.
|
|
|
|
|
Otimenyin SO, Uguru MO, Ogbonna A (2008). Antimicrobial and hypoglycemic effects of Momordica balsamina. Linn. J. Nat. Prod. 1:103-109.
|
|
|
|
|
Partap S, Kumar AS, Neeraj K, Jha KK (2012). Luffa cylindrica: An important medicinal plant. J. Nat. Prod. Pl. Res. 2(1): 127-134.
|
|
|
|
|
Pereira F, Paulo Alves Wanderley PA, Viana FAC, Baltazar de Lima R, Barbosa de Sousa F, Lima E (2011). Growth inhibition and morphological alterations of Trichophyton rubrum induced by essential oil from Cymbopogon winterianus Jowitt ex Bor. Braz. J. Microbiol. 42(1):233-242.
Crossref
|
|
|
|
|
Prashant T, Bimlesh K, Mandeep K, Gurpreet K, Harlee, K (2011). Phytochemical screening and extraction: A review. Int. Pharm. Sci. 1(10):998-106.
|
|
|
|
|
Sashikala GD, Kottai AM, Satheesh DK, Rekha S, Indhumathy NR (2009). Studies on the antibacterial and antifungal activities of the ethanolic extracts of Luffa cylindrica (Linn) fruit. Int. J. Drug Dev. Res. 1(1):105-109.
|
|
|
|
|
Scorzoni L, de Paula e Silva ACA, Marcos CM, Assato PA, de Melo WCMA, de Oliveira HC, Costa-Orlandi CB, Mendes-Giannini MJS and Fusco-Almeida AM (2017). Antifungal Therapy: New Advances in the Understanding and Treatment of Mycosis. Front. Microbiol. 8:36.
Crossref
|
|
|
|
|
Shafi S, Singh S, Haider D, Mahendhar R, Alam MS, Swamy GN, Kumar HMS (2013). Synthesis of triazole and isoxazole based novel unsymmetrical biheterocycles. J. Hetero. Chem. 50(2):361-365.
Crossref
|
|
|
|
|
Sharma BC (2012). In vitro antibacterial activity of certain folk medicinal plants from Darjeeling Himalayas used to treat microbial infections. J. Pharmacogn. Phytochem. 2(4):1-4.
|
|
|
|
|
Senguttuvan J, Paulsamy S, Krishnamoorthy K (2013). In vitro antifungal activity of leaf and root extracts of the medicinal plant, Hypochaeris radicata. Int. J. Pharm. Pharmaceut. Sci. 5(3):758-761.
|
|
|
|
|
Sofowora A (2002). Medicinal Plants and Traditional Medicine in Africa. 3rd Edn. Spectrum Books Limited, Ibadan, Nigeria, pp. 1-153.
|
|
|
|
|
Souza ACO, Amaral AC (2017). Antifungal therapy for systemic mycosis and the nanobiotechnology era: Improving efï¬cacy, biodistribution and toxicity. Front. Microbiol. 8:336.
Crossref
|
|
|
|
|
Thamaraiselvi PL, Jayanthi P (2012). Preliminary studies on phytochemicals and antimicrobial activity of solvent extracts of Eichhornia crassipes (Mart.) Solms. Asian J. Plant Sci. Res. 2(2):115-122.
|
|
|
|
|
Zohra M, Fawzia A (2011). Impact of solvent extraction type on total polyphenols content and biological activity from Tamarix aphylla (l.) Karst. Int. J. Pharm. Bio. Sci. 2(1):609- 615.
|
|


