ABSTRACT
Botrytis fabae incites chocolate spot, one of the most devastating fungal diseases infecting and constraining faba bean cultivation in Ethiopia. Culturing of B. fabae and mass production of its infective conidia has been very difficult for pathological researches on the disease caused by this fungus. Therefore, two in vitro experiments were conducted to evaluate influence of culture media on mycelial growth and sporulation of B. fabae. Ten and six separate media were evaluated for mycelial growth and sporulation, respectively. A 6 mm agar plug of fresh B. fabae culture was inoculated to each medium, arranged in CRD with 4 replications and were incubated at 22°C. Of the media tested, significantly highest (89.40 and 88.15 mm) mycelial growth were recorded on chickpea dextrose agar (CDA) and soybean dextrose agar (SBDA), respectively. Sabouraud dextrose agar supported the lowest (66.40 mm) mycelial growth 7 days after inoculation at 22°C. Significantly excellent conidial formation and spore count were recorded from Chrysanthemum flower dextrose agar (CFDA) medium (1.07 x 106/ml). Potato dextrose agar (PDA) was found inferior both in growth and sporulation of the fungus ad compared to those tested media which exhibited maximum mycelial growth and excellent sporulation. The overall results of this study indicated that cultural characteristics, mycelial growth and sporulation of B. fabae were influenced by media sources, implying that CDA and SBDA were best suited for mycelial growth. CFDA supported maximum sporulation and conidial production as compared to PDA medium. This study might be helpful for studying various aspects of B. fabae and further enhanced by complementing the tested media with different C, N and salt sources to investigate various biological and molecular characteristics of the fungus.
Key words: Botrytis fabae, conidial formation, culture media, mycelial growth, spore count.
The economic importance of faba bean (Vicia faba L.) cultivation in the world can be explained by its high nutritional value. It has been used both for human and animal consumption because of its rich protein and carbohydrate contents as well as for improving soil fertility as a green manure and nodulating crop for N2 fixation (Mathews and Marcellos, 2003; Stoddard et al., 2010). It has also significant rotational benefits predominantly in cereal-based farming systems, by suppressing cereal fungal and bacterial diseases, nematodes and weeds (Salmeron et al., 2010). However, there has been a steady reduction in the cultivated area of faba bean due to several reasons. Under the Ethiopian conditions, fungal diseases have always been the major limiting factors of faba bean cultivation. Of such fungal diseases, chocolate spot (Botrytis fabae Sard.) is highly (94.6%) prevalent in main faba bean growing areas of Ethiopia (Endale et al., 2014) and the disease caused up to 34 and 61% yield losses on tolerant and susceptible faba bean varieties, respectively (Dereje and Yaynu, 2001). Currently, a yield loss of up to 68% has been reported in northern Ethiopia (Sahile et al., 2010).
A number of physiological and environmental factors are known to influence growth and reproduction of fungi. Likewise, B. fabae requires several specific growth conditions for growth and reproduction. It is well known that growth and sporulation are important phases during the life of fungi, which is considerably influenced by external growth factors (Mishra and Tripathi, 2015). Among the external growth factors, nutrition was one of the determinants that were previously proved by several workers in many fungal pathogens using different culture media sources (Kasar et al., 2004; Rani and Murthy, 2004; Younis et al., 2004; Kim et al., 2005; Zhao et al., 2010; Lazarotto et al., 2013).
Although, only a few culture media types, which supported the radial growth, dry weight growth and sporulation of the fungus, have been reported to favor the isolation of B. fabae, the nutrient requirements for good growth of the fungus do not imply the nutrient requirements for good sporulation. Previous attempts to find suitable culture media to obtain good sporulation of B. fabae experienced difficulty (Leach and Moore, 1966; Dereje, 1993). Recently, a re-investigation on suitable media and ambient temperature levels on growth and sporulation of B. fabae has been started using semi-synthetic growth media including faba bean dextrose agar where sufficient conidial production was a problem (Terefe et al., 2015). Thus, lack of efficient culture media that support vegetative growth and sporulation of B. fabae has been an impediment to mycological and pathological studies related to the fungus. Therefore, to deal with this problem and to improve the understanding of the often different growth media requirements for growth and sporulation of B. fabae, the present investigation was undertaken to determine the best culture media type(s) for rapid mycelial growth and sporulation of B. fabae under controlled conditions.
Isolation and culturing of B. fabae
The specific B. fabae isolate used in this study was purified from naturally infected faba bean plants cultivated during the 2015/2016 main cropping season at Haramaya University Crop Research Site, Ethiopia. Faba bean infected leaflets with typical chocolate spot lesions were surface-sterilized by 5% sodium hypochlorite solution for 3 min, rinsed in sterile distilled water and dried with a sterile blotting paper. The patch specimens were placed on the surface of PDA medium in Petri dishes (ICARDA, 1986). The Petri plates with 2 to 3 mm pieces of specimens were kept in a glass case at room temperature (18 to 20°C) under 12 h day/night alternating cycles using fluorescent light and examined them five to seven days after inoculation (DAI) for emerging fungal colonies (Sahile et al., 2011). Purified cultures of the isolate were done on faba bean dextrose agar (FDA) medium at room temperature under 12 h of day/night alternating cycles of light as used by Zakrzewska (2004) and Terefe et al. (2015).
Medium preparation
With a view to find out the medium that best suits for the mycelial growth, conidia production, sporulation and other cultural characteristics of the fungus, 10 culture media, viz soybean dextrose agar (SBDA), field pea dextrose agar (FPDA), faba bean dextrose agar (FDA), potato dextrose agar (PDA), PDA complemented with faba bean meal (MnPDA), chickpea dextrose agar (CDA), chrysanthemum flower dextrose agar (CFDA), oat meal agar (OMA), sabouraud dextrose agar (SDA) and malt extract agar (MEA) in solid states were compared. Preparation procedures of each growth medium are presented as follows:
Preparation of FDA medium
200 g of faba bean seeds were rinsed in distilled water and surface-sterilized with 5% sodium hypochlorite solution for 3 min and placed into a 2 L flask. Next, 1 L of distilled water was added and the content was autoclaved in 121°C for 30 min. The resulting brew was filtered through two layers of gauze and complemented to a volume of 1 L of sterile distilled water. Next, 39 g of PDA and 5 g of agar were added and the brew was autoclaved again in 121°C for 15 min (Zakrzewska, 2004). Allowed to cool (below 50°C) and 20 ml of the medium was poured into each sterilized Petri plate.
Preparation of SBDA, FPDA and CDA media
300 g of soybean, field pea and chickpea seeds of each were rinsed in distilled water and surface-sterilized with 5% sodium hypochlorite solution for 3 min and placed into a 2 L flask. Next, similar procedures as in FDA preparation was followed.
Preparation of fresh PDA
200 g of fresh potato tubers were peeled and sliced into pieces. They were boiled in 1 L of distilled water for 40 min. The infusion was strained through two layers of cheesecloth and the potato slices were discarded. 20 g glucose and 15 g agar were added to the potato infusion and stirred together. The volume was complemented with 1 L of distilled water and autoclaved, cooled and poured to plates as in FDA preparation.
Preparation of MnPDA medium
Standard PDA medium was supplemented with faba bean seed meal (20 g of the meal per 1 L of the medium). The meal was prepared by soaking faba bean seeds in distilled water for 24 h under laboratory conditions. The imbibed seeds were heated at 90°C for 30 min and the testas were removed. The resulted seed mass was placed in flasks and autoclaved in 121°C for 30 min. Next, it was dried at a temperature between 80 and 100°C and milled (Zakrzewska, 2004; Terefe et al., 2015). The supplemented PDA was autoclaved, cooled and poured to plates as described in FDA preparation.
Preparation of CFDA medium
25 g of well dried Chrysanthemum flower was milled; and the powder was supplemented with 15 g of agar and 1 g of dextrose in 1 L of distilled water. The content was, then autoclaved, cooled and poured to plates as described in FDA preparation.
Preparation of MEA
20 g of malt extract was suspended in 1 L of distilled water and heated to boiling to dissolve the medium completely. The mix was complemented with 5 g of mycological peptone and 15 g of agar powder. The content was thoroughly mixed and sterilized, cooled and poured to plates as indicated in FDA preparation.
Preparation of SDA medium
10 g of mycological peptone was suspended in 1 L of distilled water containing 40 g of dextrose and 15 g of agar. The content was mixed and heated to boiling to completely dissolve the mix. The medium was then autoclaved, cooled and poured as in the others sections.
Preparation of OMA medium
60 g of oat meal was suspended in 1 L of distilled water. Twelve and half gram of agar was added to the mix and heated to boiling to dissolve the medium completely. The content was sterilized by autoclaving, cooled and poured to plates as described in the above sections.
Evaluation of culture media for mycelial growth of B. fabae
Mycelial plug measuring 6 mm diameter was taken from the actively growing margin of 6 day-old culture using a sterile cork borer, and placed upside down in the center of each medium in a 9 cm Petri dish. The Petri plates were immediately sealed with parafilm and arranged together in completely randomized design with four replications. All the plates were incubated at 22°C for 14 days and all the activities were done under aseptic condition inside laminar flow. Visual observations with regard to colony growth were made starting from 2 DAI. The colony diameters (mm) were measured in two directions at right angles to each other starting from 2 DAI at every 24 h interval until the mycelium fully covered the Petri dish. The colony diameter was determined by subtracting radius of centrally circular mycelial plug from the colony radius of fungal growth after each measurement. Colony morphology, shape and other cultural characteristics were observed with naked eye throughout the incubation period and characterized at full plate colony growth (7 to 11 DAI). The conidia formation was graded as: - = no sporulation, + = poor, ++ = fair, +++ = good and ++++ = excellent (Smita and Dhutraj, 2017). The experiment was repeated twice.
Evaluation of culture media for sporulation of B. fabae
Six different culture media types (MnPDA, FDA, OMA, FPDA, PDA and CFDA), which showed promising conidiation ability during the course of culture media evaluation for mycelial growth, were upgraded to this set of experiment. The efficacy of each medium on conidia production was determined by observing and counting the number of spores per ml of spore suspension. The same procedure for inoculation and incubation, as above was followed to evaluate influence of different growth culture media on sporulation of B. fabae. For spore count, 14 day-old culture of each medium was considered.
Each plate was flooded with 10 ml of sterile distilled water and its entire surface was gently rubbed with a glass rod several times to release all the spores. The spore suspension obtained was filtered through two layers of sterile gauze and was poured into a small beaker, the plate was rinsed thoroughly, and the final volume was adjusted to 20 ml by adding sterile distilled water (Lazarotto et al., 2013). For CFDA medium, being conidia dislodging with washing was not successful, the medium was suspended in 20 ml sterilized distilled water and gently milled with the help of laboratory blender to ensure complete detachment of spores. The solution was filtered through two layers of sterile gauze to discard mycelial fragments. Sporulation was determined under the microscope by counting 4 samples per replicate. The number of spores was counted and measured using the Malassez haemocytometer slide under an optical microscope field of vision (10x eyepiece and 40x objective).
Statistical data analysis
Data from two runs of experiments were pooled after confirming homogeneity of variances for growth and sporulation evaluation. Analysis of variance was performed to determine influence of different culture media sources on mycelial growth rate and sporulation of B. fabae using SAS GLM Procedure (SAS Institute, 2001). The numbers of conidia per milliliter were analyzed after logarithmic transformation of the values obtained (Pefoura et al., 2007). Mean separations were made using the least significant difference (LSD) test at 0.05 probability level. Regression analyses of diameters of colony radial growth against time after inoculation were performed and the slopes were used as measures of growth rates (mm day-1) for each culture medium source treatment (Ramirez et al., 2004). Bartlett's variance homogeneity tests were performed for each variable before combining data over the two runs of experiments both in radial growth and sporulation studies (Gomez and Gomez, 1984).
Effect of culture media on mycelial growth of B. fabae
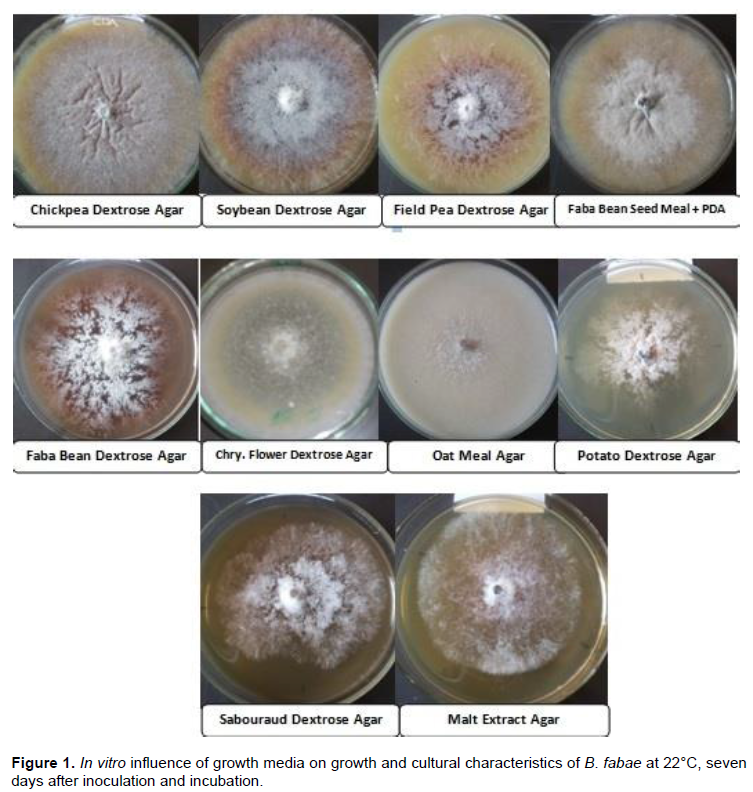
All tested culture media supported growth of the fungus to various degrees and the culture media significantly (P ≤ 0.05) affected mycelial growth of B. fabae (Table 1). The mean colony diameter measured with the entire test media ranged from 66.40 (on SDA) to 89.40 mm (on CDA) at 7 DAI. Among the tested culture media sources, CDA (89.60 mm) and SBDA (88.15 mm) medium showed statistically similar mycelial growth and both were found to be more suitable for vegetative growth than the other culture media. While, MnPDA (85.40 mm) and FDA (84.53 mm) also showed comparably maximum growth as the most suitable ones. The third group of culture media included OMA (81.90 mm), FPDA (81.65 mm) and CFDA (81.15 mm) produced moderate mycelial growth and they were statistically on par with each other. The rest culture media had mycelial growth of the fungus ranging from 66.40 to 77.78 mm. One of the most commonly exploited culture media for growth of many fungal pathogens, PDA, supported the third least mycelial growth (77.78 mm) of the fungus under study.
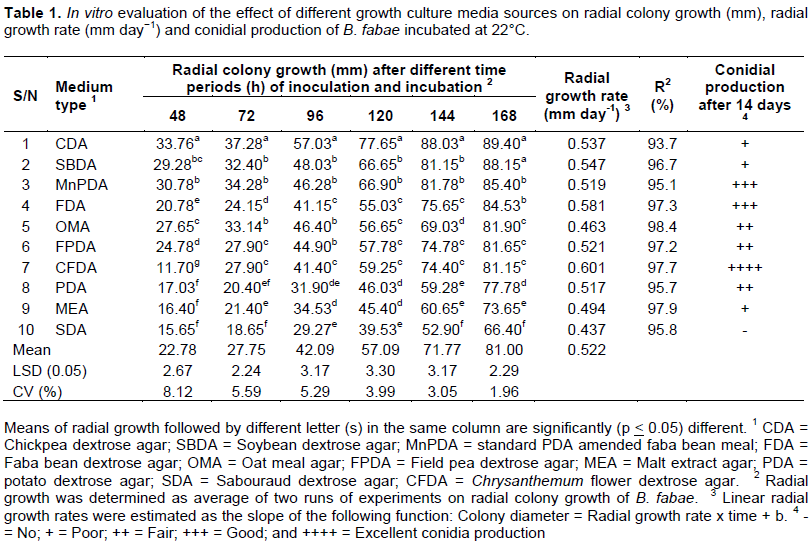
Fresh culture media source complements prepared from chickpea, soybean, faba bean and field pea further promoted mycelial growth from moderate to higher mycelial growth of the fungus than on synthetic culture media sources tested. The fungus displayed a growth rate ranging from 0.437 to 0.601 mm day-1 where a slower (0.437 mm day-1) growth rate was calculated on SDA medium than on the other culture media sources tested at 22°C. Thus, radial growth rate was affected by variation in culture media sources. At the tested temperature, mycelial growth on CDA and SBDA media reached the edge of the Petri plate about seven DAI and incubation, implying that mycelial growth of B. fabae was culture media dependent.
Effect of culture media on sporulation of B. fabae
There was significant variation in spore density per culture medium used and culture media significantly (P < 0.05) affected conidia production and sporulation of B. fabae (Table 2). Among the six culture media sources upgraded and tested for conidiation and sporulation, excellent (1.07 x 106 spores/ml) spore count of the fungus was observed on CFDA medium at 14 DAI and incubation, which was significantly different from the other tested media. In order of suitability for sporulation, CFDA was followed by MnPDA (2.61 x 105 spores/ml) and FDA (2.42 x105 spores/ml), which showed good degree of sporulation of the fungus. The rest three culture media sources including OMA (6.2 x 104 spores/ml), FPDA (5.1 x 104 spores/ml) and PDA (7.0 x 104 spores/ml) were found to be less favorable for sporulation of the fungus than the other culture media where their degree of sporulation was only fair (++) on the same date after inoculation and incubation. This implying that sporulation of B. fabae was also culture media dependent.
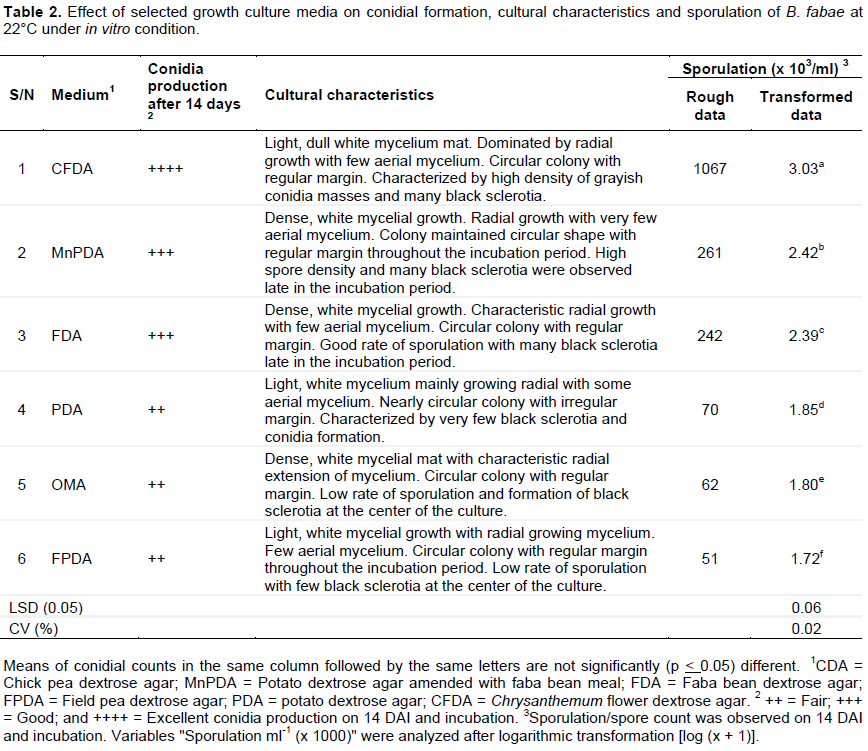
Observations on various colony characters were also recorded. All the culture media exhibited a wide range of colony characteristics with regard to conidial and sclerotial formation, rate of sporulation, shape and mycelial growth habit (Table 2 and Figure 1). Mycelial growth patterns included both light and dense radial extending mycelium. Radial mycelial growth was consistently a characteristic feature of the fungus on all media sources. Highly dense, white mycelia and circular colonies with regular margins were observed on OMA, MnPDA and FDA culture media, whereas light, white mycelia and circular colonies with more or less regular margins were recorded on PDA, FPDA and CFDA culture media. It was also observed that all the tested culture media sources exhibited different levels of black sclerotia formation and distribution at 22°C late in the incubation period.
Searching for alternative suitable culture media for growth, sporulation and cultural characteristic studies of the pathogen is the first step in pathological research. In this study, culture media strongly influenced growth, sclerotial and conidial formation, and sporulation of B. fabae. Several studies also found similar results with regard to the effect of culture media on growth, sporulation and other cultural characteristics of various types of fungi (Jackson and Bothast, 1990; Kim et al., 2005; Zhao et al., 2010; Ibrahim and Shehu, 2015; Koley and Mahapatra, 2015; Mishra and Tripathi, 2015).
Chickpea dextrose agar and SBDA culture media, which supported the maximum radial growth rate, were found to be the most suitable for mycelial growth of B. fabae. On the other hand, the most commonly used medium, PDA, supported moderate mycelial growth of the fungus. This could be attributed to the variation in the concentration of C, N and other nutrients in each medium tested. Fresh media preparations from chickpea, soybean, field pea and faba bean contained high concentration of C and N sources, which supported maximum mycelial growth of the pathogen. Previous research studies also indicated in their studies with various types of fungi that high concentration of C and N induces vegetative and abundant mycelial growth (Saha et al., 2008; Zhao et al., 2010; Mishra and Tripathi, 2015; Koley and Mahapatra, 2015).
B. fabae grew slowly on PDA in contrast to the findings of Kim et al. (2005) who concluded that PDA supported maximum mycelial growth of S. pyriputrescens and several other researchers studied on many fungi (Maheswari et al., 1999; Kumar and Singh, 2000; Alam et al., 2001; Unagul et al., 2005; Saha et al., 2008; Premalatha et al., 2012; Pradeep et al., 2013), but this finding was in proximity to the mycelial growth of Diplocarpon mali, which attained only < 10 mm on PDA medium after one month of incubation (Zhao et al., 2010), implying that PDA is not an ideal culture medium for the growth and sporulation of B. fabae.
Chrysanthemum flower dextrose agar resulted in excellent and abundant conidial formation and sporulation of the test fungus. MnPDA and FDA supported average conidiation ability and sporulation of B. fabae, whereas the rest tested culture media showed fair (OMA, FPDA and PDA), poor (CDA, SBDA and MAE) and no (SDA) sporulation. Those fresh culture media sources supporting higher mycelial growth than others were found to contain too much nutrients and inhibited sporulation. A similar study by Saha et al. (2008) indicated that PDA and other culture media having good sugar content allowed the best mycelial growth of A. solani, but too much nutrients leads to loss of sporulation (UKNCC, 1998). A study by Koley and Mahapatra (2015) also pointed out that OMA supported good sporulation of A. solani as compared to PDA due to lower sugar contents than PDA. Heavy perithecia was formed by C. funicola on LCA medium having low glucose content (Sharma and Pandey, 2010). Low glucose content is known to suppress the overgrowth of fast growing species of fungi and induces sporulation (Osono and Takeda, 1999). What was found in this current study is also in parallel with the above findings in which CFDA contained low sugar levels that supported sporulation of B. fabae.
B. fabae grew well on all the 10 culture media employed in the study, while conidiated and sporulated on eight of the tested culture media. However, the best culture media for mycelial growth were CDA and SBDA, while MnPDA and FDA supported good sporulation of the fungus. But, CFDA is recommended for mass production of conidia during conidial characteristic and artificial inoculation studies, both culture media types suited for mycelial growth, and conidiation and sporulation could be employed for other cultural characteristic studies of B. fabae as well. This implies that a combination of two or more culture media would be more appropriate for cultural studies of the fungus than using any single medium. Thus, it is recommended that this information generated from this study can be used in mycological and pathological researches dealing with B. fabae, cause chocolate spot of faba bean. This study might be further enhanced by complementing the tested culture media with variable and known C, N and salt sources to investigate various biological and molecular characteristics of the fungus.
The authors have not declared any conflict of interests.
REFERENCES
|
Alam MS, Begum MF, Sarkar MA, Islam MR, Alam MS (2001). Effect of temperature, light and media on growth, sporulation, formation of pigments and pycnidia of Botryodiplodia theobromae. Pak. J. Biol. Sci. 4(10):1224-1227.
Crossref
|
|
|
|
Dereje G (1993). Studies on the epidemiology of chocolate spot (Botrytis fabae Sard.) of faba bean (Vicia faba L.). M.Sc. Thesis, Alemaya University of Agriculture. Alemaya, Ethiopia. pp. 30-70.
|
|
|
|
|
Dereje G, Yaynu H (2001). Yield loss of crops due to plant diseases in Ethiopia. Pest Manag. J. 5:55-67.
|
|
|
|
|
Endale H, Gezahegn G, Tadesse S, Nigussie T, Beyene B, Anteneh B, Daniel K, Tamene T (2014). Faba bean gall: a new threat for faba bean (Vicia faba) production in Ethiopia. Adv. Crop. Sci. Technol. 2:1-5.
|
|
|
|
|
Gomez KA, Gomez AA (1984). Statistical procedures for agricultural research. (2nd edn). John Wiley and Sons Inc., New York. pp. 458-478.
|
|
|
|
|
Ibrahim M, Shehu K (2015). Effects of Various carbohydrates on the growth of Rhizopus stolonifer. Int. J. Multidiscip. Res. Dev. 2(5):306-308.
|
|
|
|
|
ICARDA (International Center for Agricultural Research in the Dry Areas) (1986). Screening techniques for disease resistance in faba beans. International Center for Agricultural Research in the Dry Areas (ICARDA), Aleppo, Syria. pp. 1-59.
|
|
|
|
|
Jackson MA, Bothast RJ (1990). Carbon concentration and carbon-to-nitrogen ratio influence submerged-culture conidiation by the potential bioherbicide Colletotrichum truncatum NRRL 13737. Appl. Environ. Microbiol. 56(11):3435-3438.
|
|
|
|
|
Kasar P, Maity SS, Bhattacharya R, Chowdhury AK, Khatua DC (2004). Occurrence of guava fruit canker in West Bengal and bioassay of fungicides against pathogen. Horticulture 17:219-225.
|
|
|
|
|
Kim YK, Xiao CL, Rogers JD (2005). Influence of culture media and environmental factors on mycelial growth and pycnidial production of Sphaeropsis pyriputrescens. Mycologia 97:25-32.
Crossref
|
|
|
|
|
Koley S, Mahapatra SS (2015). Evaluation of culture media for growth characteristics of Alternaria solani, causing early blight of tomato. J. Plant Path. Microbiol. S1:005.
|
|
|
|
|
Kumar PS, Singh SP (2000). First report of Lasiodiplodia theobromae as a foliar pathogen of Parthenium hysterophorus. Plant Dis. 84(12):1343.3-1343.3.
|
|
|
|
|
Lazarotto M, Mezzomo R, Maciel CG, Finger G, Muniz MFB (2013). Mycelial growth and sporulation of Fusarium chlamydosporum species complex under different culture conditions. Amaz. Agric. Environ. Sci. 57(1):35-40.
|
|
|
|
|
Leach R, Moore KG (1966). Sporulation of Botrytis fabae on agar cultures. Trans. Br. Mycol. Soc. 49(4):593-601.
Crossref
|
|
|
|
|
Maheswari SK, Singh DV, Sahu AK (1999). Effect of several nutrient media, pH and carbon sources on growth and sporulation of Alternaria alternata. J. Mycopathol. Res. 37:21-23.
|
|
|
|
|
Mathews P, Marcellos H (2003). Faba Bean. 2nd Edition. Agfact, P4.2.7. NSW Agriculture. pp. 1-12.
|
|
|
|
|
Mishra NK, Tripathi BP (2015). Effect of culture media on growth, colony character and sporulation of three foliar pathogens of guava. The Bioscan. 10(4):1701-1705.
|
|
|
|
|
Osono T, Takeda H (1999). A methodological survey on incubation of fungi on leaf litter of Fagus crenata. Appl. For. Sci. Kansai. 8:113-103.
|
|
|
|
|
Pefoura AM, Ouamba AJK, Nkenfou C, Nguidjo O, Dongmo R (2007). Influence of the temperature on radial growth and sporulation of Trachysphaera fructigena, causal agent of the Musa cigar end rot disease. Afr. Crop Sci. Con. Proc. 8:849-852.
|
|
|
|
|
Pradeep FS, Begam MS, Palaniswamy M, Pradeep BV (2013). Influence of culture media on growth and pigment production by Fusarium moniliforme KUMBF1201 isolated from paddy field soil. World Appl. Sci. J. 22 (1):70-77.
|
|
|
|
|
Premalatha B, Pradeep FS, Pradeep BV, Palaniswamy M (2012). Production and characterization of naphthoquinone pigment from Fusarium moniliforme MTCC6985. World J. Phar. Res. 1:1126-1142.
|
|
|
|
|
Ramirez ML, Chulze SN, Magan N (2004). Impact of osmotic and matric water stress on germination, growth, mycelial water potentials and endogenous accumulation of sugars and sugar alcohols in Fusarium graminearum. Mycol. 96:470-478.
Crossref
|
|
|
|
|
Rani SG, Murthy KVMK (2004). Cultural and nutritional characteristics of Colletotrichumgloeosporioides, the causal organism in cashew anthracnose. J. Mycol. Plant Pathol. 34:317-318.
|
|
|
|
|
Saha A, Mandal P, Dasgupta S, Saha D (2008). Influence of culture media and environmental factors on mycelial growth and sporulation of Lasiodiplodia theobromae (Pat.) Griffon and Maubl. J. Environ. Biol. 29(3):407-410.
|
|
|
|
|
Sahile S, Chemeda F, Sakhuja PK, Seid A (2010). Yield loss of faba bean (Vicia faba) due to chocolate spot (Botrytis fabae) in sole and mixed cropping systems in Ethiopia. Arch. Phytopathol. Plant Prot. 43:1144-1159.
Crossref
|
|
|
|
|
Sahile S, Sakhuja PK, Fininsa C, Ahmed S (2011). Potential antagonistic fungal species from Ethiopia for biological control of chocolate spot disease of faba bean. Afr. Crop Sci. 19:213-225.
|
|
|
|
|
Salmeron JIC, Avila C, Torres AM (2010). Faba bean and its importance in food security in developing countries. 13p. In: Solh, M. and Saxena, M.C. (eds.), Food security and climate change in dry areas. International Conference 1-4 February 2010. Amman, Jordan.
|
|
|
|
|
SAS Institute (2001). SAS/STAT Users Guide, Version 8.2. SAS Institute Inc., Cary, NC, USA.
|
|
|
|
|
Sharma G, Pandey RR (2010). Influence of culture media on growth, colony character and sporulation of fungi isolated from decaying vegetable wastes. J. Yeast Fungal Res. 1(8):157-164.
|
|
|
|
|
Smita C, Dhutraj DN (2017). Effect of culture media and temperature on growth and sporulation of Colletotrichum truncatum of soybean in vitro. Int. J. Appl. Pur. Sci. Agri. 3(1):25-30.
|
|
|
|
|
Stoddard FL, Nicholas AH, Rubiales D, Thomas J, Villegas-Fernández AM (2010). Integrated pest management in faba bean. Field Crops Res. 115:308-318.
Crossref
|
|
|
|
|
Terefe H, Fininsa C, Sahile S, Tesfaye K (2015). Effect of temperature on growth and sporulation of Botrytis fabae, and resistance reactions of faba bean against the pathogen. J. Plant Pathol. Microbiol. 6: 285.
|
|
|
|
|
UKNCC (The United Kingdom National Culture Collection) (1998). Growth and media manuals. Strain databases. Accessed from
View
|
|
|
|
|
Unagul P, Wongsa P, Kittakoop P, Intamas S, Srikitikulcha PI, Tantichareon M (2005). Production of red pigment by insect pathogenic fungus Cordyceps unilateralis, BCC 1869. J. Ind. Microbiol. Biotechnol. 32:135-140.
Crossref
|
|
|
|
|
Younis M, Khalid-Mehmood, Rashid A, Waseem MA (2004). Physiological studies on Pestalotiapsidii and its control. Int. J. Agric. Biol. 6:1107-1112.
|
|
|
|
|
Zakrzewska E (2004). Reaction of morphological types of faba bean to infection with Ascochyta fabae Speg. and Botrytis fabae Sard. Plant Breed. Seed Sci. 49:3-7.
|
|
|
|
|
Zhao H, Huang L, Xiao CL, Liu J, Gao X (2010). Influence of culture media and environmental factors on mycelial growth and conidial production of Diplocarpon mali. Lett. Appl. Microbiol. 50:639-644.
Crossref
|
|