ABSTRACT
Antimicrobial drug residues have emerged as one of the public health problems worldwide. In this study, a modified sensitive liquid chromatography mass spectrometry (LC-MS) method to detect the “Oxytetracycine” (OTC) levels in ready-to-eat beef meat in Tanzania was evaluated. Beef samples were extracted in acetonitrile in ethylenediaminetetraacetic acid (EDTA) buffer (pH 4), followed by cleaning up with Supelclean ENVI-carb active coal and a stream of nitrogen gas. The wavelength of the diode array detector (DAD) was set at 275 and 355 nm. The detection limit of the method was calculated as 18.2 ng/g and the recovery rate of OTC was 78.6%. A total of 45 ready-to-eat beef meat samples were analyzed, with 16 (35.5%) and 29 (64.5%) barbequed and boiled samples, respectively. Of the 45 samples, 35 (77.8%) samples had OTC residues while 9 (25.7%) samples had violative residue levels above the maximum residue limits recommended by the Food and Agriculture Organization and the World Health Organization. The highest concentration was 545.2 ng/g. Therefore, withdrawal period and proper use of antibiotics for animal production should be of concern as consumers are at risk of adverse effects due to consumption of unacceptable levels of drug residues and a risk of developing microbial resistance. To the best knowledge of the authors, this is the first study to evaluate LC-MS method to detect the OTC levels in ready-to-eat beef meat in Tanzania.
Key words: Oxytetracycline, high performance liquid chromatography, mass spectrometry, ready-to-eat beef meat, residues.
Antimicrobial drug residue in animal products is an increasing public health problem worldwide. One of the major areas of interest is investigating the proper use and monitoring of antibiotics usage to prevent contamination (Alica et al., 2003). Questions have been raised about the drug label, discard times as several drugs are retained in animal bodies
longer than indicated by the manufacturer (Seymour et al., 1988). Improper administration of antimicrobials by farmers and veterinarians without observing the withdrawal time for treated animals may not only result in antimicrobial residues in meat but may also contribute to the development of microbial drug resistance and spreading of drug resistant bacteria that may result in serious health consequences (Booth, 1988). Human health problems that could arise from the consumption of unacceptable levels of OTC residues in meat include gastrointestinal disturbances, hyper-sensitivity, bone and teeth problems in children and development of bacterial resistance (Larkin et al., 2004; Shankar et al., 2010).
The problem regarding tetracycline residues is very common and has to be addressed accordingly, since tetracyclines are the commonly used antimicrobial drugs. With this regard the Food and the Agriculture Organization (FAO) and the World Health Organization (WHO), 2004 recommended the maximum residue limits (MRLs) to be 200, 600 and 1200 μg/kg in muscles, livers and kidneys, respectively. For the analysis of tetracyclines levels, various methods have been reported in the literature mainly due to difficulties related to differences in physico-chemical properties between families of compounds (Kaufmann, 2009). Methods for the detection of tetracyclines are many but a more specific method such as HPLC is the efficient technique (Loksuwan 2002; Cinquina et al., 2003). The method efficiency is based on multi-detection on liquid chromatography coupled with tandem mass spectrometry (Bohm et al., 2009).
Residues are ordinarily measured on uncooked tissues. It is also important to monitor the levels of drug residues in both raw and ready-to-eat foodstuffs. Studies have shown that temperatures have effect on the levels of drug residues (Salah and Ali, 2013). It is even more important to analyse the levels of OTC residues and to evaluate if residues levels can be reduced by cooking procedures (Ibrahim and Moats, 1994). So far, there is limited literature about the effect of cooking on levels of residues and this creates a scientific gap of knowledge which needs to be addressed in Tanzania. Therefore, the objective of the present work was to modify and validate a simple and sensitive LC-MS method for analyzing Oxytetracycine (OTC) residues (Froehlich, 2013). The validated method was applied to determine the levels of OTC in ready-to-eat beef meat samples.
Samples
A total of 45 ready-to-eat beef meat samples were randomly collected from different areas in Dodoma, Tanzania (Majengo Sokoni, Mnadani, Chakonichako, Rozi Garden and Bahama Mama). The samples collected were already prepared as barbequed ‟nyama choma” or boiled. These two methods of preparation were selected because they are most practiced in Tanzania. Antibiotics-free meat samples (blank matrix) were collected from the Central Veterinary Research Institute of Zambia. The blank matrix samples were barbaqued or boiled before extraction.
Sample pretreatment and extraction
The samples were kept at -20°C until analysis and were allowed to defrost at room temperature. A representative portion of the defrosted sample (10 g) was weighed and mixed with 25 mg of EDTA per gram sample. The sample and the EDTA were homogenized for 1 min using a blender. The blended sample was further ground using a mortar and pestle. One gram of homogenized sample was accurately weighed into 15 ml polypropylene centrifuge tubes. To the sample, 10 µl of 10 µg/ml carbamazepine D10 internal standard solution equivalent to 100 ng/g concentration was added.
Five milliliters acetonitrile were added to the sample and vortexed for 1 min. Each sample was centrifuged for 10 min at 7000 rpm and the supernatant was collected into a separate 15 ml centrifuge tube by decantation. 5 ml acetonitrile were again added to the residue and vortexed for 1 min. The samples were then centrifuged for 10 min at 7000 rpm. Both supernatants were combined in a 15 ml centrifuge tube bringing the total volume to 10 ml. All samples were briefly mixed using a vortex and dried under a stream of nitrogen gas to 2 ml, according to Froehlich's HPLC method (Froehlich, 2013).
Sample clean-up by Supelclean ENVI-carb active coal
After drying each sample to 2 ml, 0.5 ml of HPLC grade water and 30 µl of formic acid were added, making the mixture 1.2% acid. Then 15 mg of Supelclean ENVI-carb active coal was added to all the samples and mixed for 30 s using a
vortex and centrifuged for 10 min at 7000 rpm. The supernatants were collected into separate 15 ml centrifuge tubes and dried to 0.5 ml. The dried samples were then transferred into HPLC vials washed with 0.02 mol/L EDTA solutions and injected into chromatographic system (Froehlich, 2013). The HPLC analysis was performed in 23 min.
Sample analysis by LC-MS method
The HPLC was equipped with DAD detector and mass spectroscopy (Model Agilent Technologies 6130 Quadrupole LC/MS) to target the flowing parent ions using Single Ion Monitoring (SIM) mode 461 mass per charge ratio (m/z) for OTC. The analytical column was reversed-phase Eclipse XDB C-18. 4.6 × 150 mm set at a flow rate of 0.5 ml/min. The column temperature was 25°C. Mobile phase A was HPLC water with 0.1% formic acid and solvent C was Acetonitrile with 0.1% formic acid. The starting mobile phase composition at 0 min was 85% Water: 15% Acetonitrile at 0.5 ml/min. The wavelength of the DAD detector was set at 275 and 355 nm, respectively. Internal calibration curves were prepared by spiking the blank matrix with pure chromatographic standard solutions in the range between 200 and 2500 ng/g injected for each compound and estimates of the amount of the analytes in samples were interpolated from these graphs.
Validation
To test the analytical method trueness, 14 samples were prepared. Each contained 1 g of homogenized muscle tissue of the negative control sample (blank matrix). Seven samples were spiked with 20 µl of 10 ng/ml solutions, equivalent to 200 ng/g of analyte. Seven samples were spiked with 250 µl equivalent to 2500 ng/g of the analyte. All samples were processed using the described LC-MS method.
Preparation of standard stock and working solution
A stock standard solution of OTC compound was prepared by dissolving 10 mg of the compound in 10 ml of methanol to obtain a final concentration of 1 mg/ml. The stock standard solution was then put in amber glasses to prevent photo-degradation and stored at -20°C and left to stabilize for at least 4 weeks. They were then diluted with 95% water: 5% acetonitrile to give a series of working standard solution of 200, 400, 800, 1200, and 2500 ng/g.
Recovery experiment
Samples recovery was determined with blank bovine muscle spiked at 200 ng/g. To test the recovery, 10 samples were prepared that contained 1 g of homogenized muscle tissue of the negative control. They were spiked with 20 µl of 10 ug/ml spiking solution equivalent to 200 ng/g of the analyte. Four samples were used to calculate the recovery mean and six samples were used to calculate the recovery-corrected content.
Data analysis
The data were analyzed using Epi Info (version 7) (Centre for Disease Control, Atlanta, USA). The association between different categorical and continuous variables was determined by the Fisher’s exact test. One-way analysis of variance (ANOVA) test statistic was used to determine any significant differences in the mean residue levels of oxytetracycline; a probability of P < 0.5 was considered statistically significant.
Calibration of OTC standard
OTC standard powder was accurately weighed and dissolved in methanol to make the stock solution and several serial dilutions of the stock solution were made and injected to the LC-MS to plot the standard curve of linear R² value = 0.9971 within the range of 200 to 2500 ng/g (Figure 1).
Samples recovery
The recovery rate of OTC was 68% (Table 1), while the recovery-corrected rate for the samples were 78.6% ranging from 64.8 to 86.9% (Table 2). For repeatability and reproducibility, data were obtained by extracting 7 replicates on three successive days at two concentrations of 200 and 2500 ng/g; with coefficients of variation of 6.60 to 10.60% and 6.30 to 10.60% for OTC, respectively. Results of this study revealed that the repeatability and reproducibility were corresponding to the validation methods done by Biswas et al. (2007).
LC-MS technique was employed to determine the levels of OTC in ready-to-eat beef meat samples in Dodoma, Tanzania. In this method, carbamazepine D10 was used as internal standard to correct internal and external error.
The detection of OTC residues levels was done by using LC method with MS detector. This is because OTC can be successfully determined using LC with MS detector in various matrices. Adequate treatment of samples during extraction was done in order to obtain maximum sensitivity of OTC and to reduce matrix interference. The samples were considered positive for OTC if their retention time and peak corresponded to that of the reference standard. The retention time of the standard was at 3.624 min. The chromatographic peak increased with increase in concentration of the standard
The limit of detection (LOD) is the lowest concentration which can be qualitatively measured, and is deï¬ned as the concentration at which the signal-to-noise ratio of the corresponding signal is 3-to-1. In this study, the LOD was 18.2 ng/g, corresponding to the LOD obtained by Hassani et al. (2008). The limit of quantiï¬cation (LOQ) is the lowest concentration of analyte which can be quantitatively measured and was 54.6 ng/g.
Figure 2 shows LC-MS profiles of the OTC obtained from the blank beef meat samples, blank beef samples spiked with 400ng OTC, standard solution and spiked beef meat samples.
Figure 2. LC-MS profiles of OTC. (AUC = Area under the curve). (a) Chromatogram of blank beef meat sample. (b) Chromatogram of blank beef samples spiked with 400ng OTC. (c) Chromatographic standard solution. (d) Chromatogram of spiked beef meat sample of positive OTC thermally treated.
Results indicate that of the 45 beef meat samples analyzed 16 (35.5%) were barbequed samples and 29 (64.5%) boiled samples. The observed differences are statistically insignificant (P > 0.05) as shown in Table 3.
Thirty five samples (77.8%) had OTC residues with 26 (74.3%) samples having residues below the FAO/WHO (2004) recommended MRLs. Nine (25.7%) samples had OTC at violative levels above the recommended MRLs. Of the 9 samples with detectable violative OTC levels, 2 (22.2%) and 7 (77.8%) samples were barbequed and boiled meat samples, respectively. However, the observed differences were statistically insignificant (P > 0.05) as shown in Table 4.
The study findings indicate the need for one health strategy to enhance the optimal health for humans, animals and the environment.
Mean concentration of OTC residues in barbequed and boiled samples were 130.67 ± 96.6 and 361.96 ± 69.40 µg/kg, respectively. The concentration of OTC residues from each sample is shown in Table 5. This study shows higher proportions of oxytetracycline–positive samples than those reported in other studies (Addisalem et al., 2012) and Bedada and Zewde (2012). Studies have reported varied drug residues in raw meat samples, 41.2% (Mmbando, 2004) and 76.4% (Nonga et al., 2013). in Tanzania. Nevertheless, the study conducted by Mmbando (2004) from muscle tissue in the Morogoro and Dodoma municipalities, Tanzania, indicate that only 41.2% of samples were positive for oxytetracycline residues. Drug residues in raw meat have also been reported in other countries, 44% in Nigeria (Stolker and Brinkman, 2005), 50% in Iraq (Tajick and shohreh, 2006), 21% in Ghana (Donkor et al., 2011) and 71.3% in Ethiopia (Addisalem et al., 2012). From Ghana, Donkor et al. (2011) and Mmbando (2004) reported 21 and 41.2% oxytetracycline residues in muscle tissue were relatively low compared to levels seen in the current study. These results reported here are consistent with those previously reported by Nonga et al. (2013) from Tanzania and those by Addisalem et al., (2012) from Ethiopia of 76.4 and 71.3%, respectively.
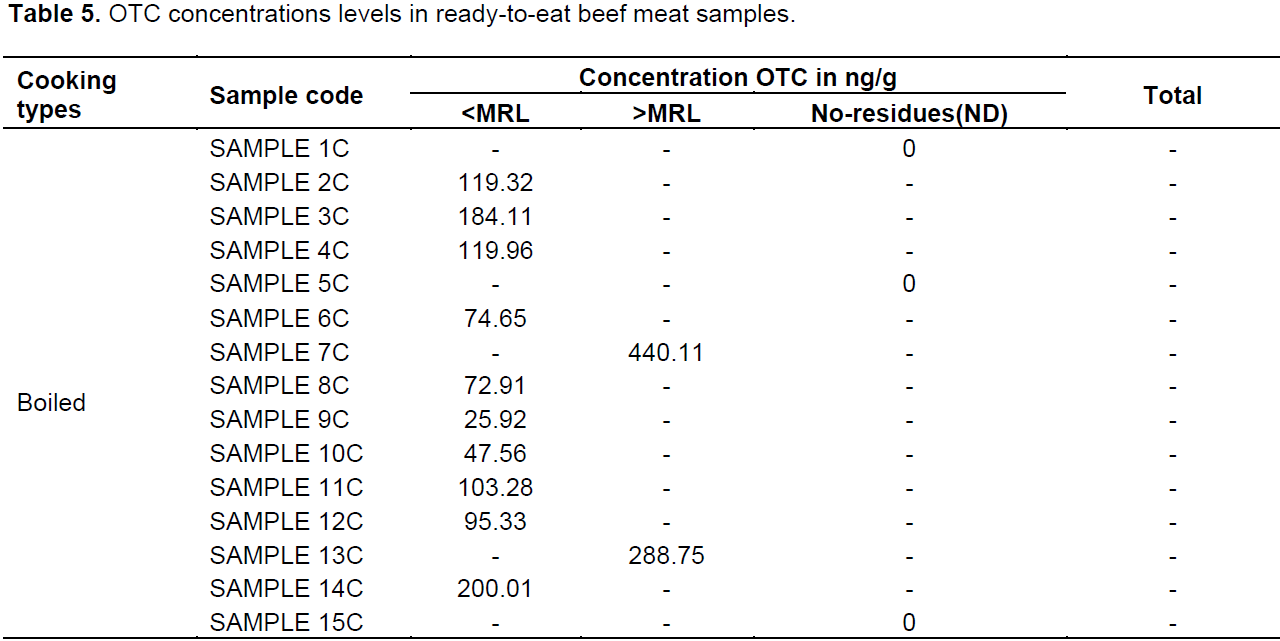
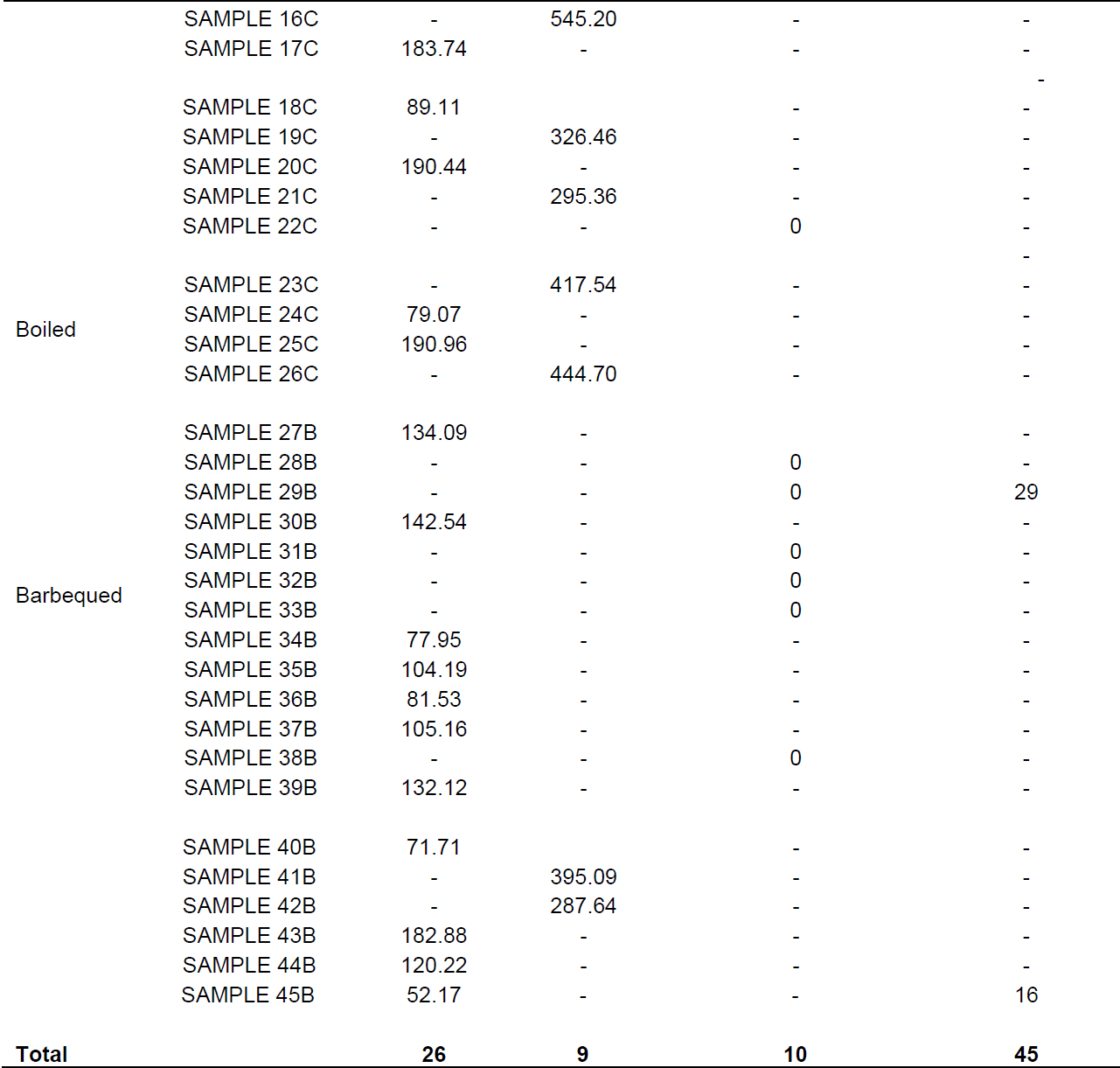
The presence of OTC residues in the ready-to-eat meat observed in the present study is a clear indication that drug residues are not destroyed by heating/cooking. The reasons might be due to the method used, time of cooking and type of tetracycline (TC) used. Several studies reported the effect of heat on foodstuffs. Nguyen et al. (2013) have reported that heat treatments were shown to reduce the concentration of drug residues level in foodstuffs, therefore decreasing the toxic effects to consumers. Javadi (2011) and Gratacós-Cubarsí et al. (2007) showed reductions in the concentration of doxycline (DOC) and OTC residues level after different cooking processes. A study by Al-Ghamdi et al. (2000) also indicated that cooking by boiling decreased OTC, Chlortetracycline (CTC) and DOC levels in meat and liver.
A simple, rapid and sensitive LC-MS method for the detection of OTC levels in beef meat samples was evaluated. The method was capable of detecting residue and non-residue meat samples. A significant proportion of ready-to-eat beef meat samples (25.7%) had OTC level above the FAO/WHO MRLs of 200 μg/kg. This indicates that animals are slaughtered without giving adequate withdrawal period or misuse of antibiotics for animal production in Dodoma region, Tanzania. The consumers of ready-to-eat beef meat are at risk of adverse effects due to consumption of unacceptable levels of drug residues and a risk of developing microbial resistance.
The study findings signify the need for the One Health approach for effective surveillance of drug residues in foodstuffs. Therefore, withdrawal period and proper use of antibiotics for animal production should be a public health concern given that the One Health approach aims to attain the optimal health for humans, animals and the environment. To the best knowledge of authors, this is the first study to evaluate LC-MS method to detect the OTC levels in ready-to-eat beef meat in Tanzania.
The authors have declared that they have no conflict of interests.
The authors are grateful to INTRA-ACP MOBILITY for funding this study and the University of Zambia and the Zambia Agriculture Research Institute for allowing the use of their research facilities during the study period.
REFERENCES
|
Addisalem HB, Bayleyegn MZ, Bayleyegn MZ (2012). Tetracycline residue levels in slaughtered beef cattle from three slaughterhouses in Central Ethiopia. Glob. Vet. 8(6):546-554.
|
|
|
|
Al-Ghamdi MS, Al-Mustafa ZH, El-Morsy F, Al-Faky A, Haider I, Essa H (2000). Residues of tetracycline compounds in poultry products in the eastern province of Saudi Arabia. J. Pub. Health 114:300-304.
Crossref
|
|
|
|
|
Alica D, Jennifer M, Shannon R, Frederick J (2003). Public health consequences of use of antimicrobial agents in food animals in the United States. J. Microbiol. Drug Resist. 9:1-7.
|
|
|
|
|
Bedada AH, Zewde BM. (2012). Tetracycline residue levels in slaughtered beef cattle from three slaughterhouses in central Ethiopia. J. Glob. Vet. 8(6):546-554.
|
|
|
|
|
Biswas AK, Rao GS, Kondaiah N, Anjaneyulu SR, Mendiratta SK, Prasad R, Malik JK (2007). A Simple Multi-residue Method for Determination of Oxytetracycline, Tetracycline and Chlortetracycline in Export Buffalo Meat by HPLC-Photodiode Array Detector. J. Food Drug Anal. 15(3):278-284.
|
|
|
|
|
Bohm DA, Stachel CS, Gowik P (2009). Multi- method for the determination of antibiotics of different and substance groups in milk and validation in accordance with Commission Decision 2002/657/EC. J. Chromatogr. A. 1216:8217-8223.
Crossref
|
|
|
|
|
Booth NH (1988). Toxicology of drug and chemical residues in Veterinary Pharmacology and Therapeutics. Iowa State University Press, pp. 1149-1205.
|
|
|
|
|
Cinquina AL, Longo F, Anastasi G, Giannetti L, Cozzani R (2003). Validation of a high-performance liquid chromatography method for the determination of oxytetra-cycline, tetracycline, chlortetracycline and doxycycline in bovine milk and muscle. J. Chromatogr. A. 987(1-2):227-233.
Crossref
|
|
|
|
|
Donkor ES, Newman MJ, Tay SCK, Dayie NT, Bannerman E, Olu-Taiwo M (2011). Investigation into the risk of exposure to antibiotic residues contaminating meat and egg Ghana. J. Food Control. 22:869-873.
Crossref
|
|
|
|
|
FAO/WHO (2004). Residues of some veterinary drugs in animals and foods. Sixty-second report of the Joint FAO/WHO export committee on food additives. WHO Technical Report Series. FAO FNP 41/16.
|
|
|
|
|
Froehlich B (2013). Development of a LC-UV/Vis-FLD method for the quantification of Sulfamethazine, Tetracycline, Oxytetracycline and Chlortetracycline in poultry meat. J. Assoc. Anal. Chem. 61(5):1222-1227.
|
|
|
|
|
Gratacós-Cubarsí M, Fernandez-García A, Picouet P, Valero-Pamplona A, García-Regueiro J, Castellari M (2007). Formation of tetracycline degradation products in chicken and pig meat under different thermal processing conditions. J. Agric. Food Chem. 55:4610-4616.
Crossref
|
|
|
|
|
Hassani M, Lázaro R, Pérez C, Condón S, Pagán R (2008). Thermostability of oxytetracycline, tetracycline and doxycycline at ultrahigh temperatures. J. Agric. Food Chem. 56:2676-2680.
Crossref
|
|
|
|
|
Ibrahim A, Moats WA (1994). Effect of cooking procedures on oxytetracycline residues in lamb muscle. J. Agric. Food Chem. 42:2561-2563.
Crossref
|
|
|
|
|
Javadi A (2011). Effect of roasting, boiling and microwaving cooking method on doxycline residues in edible tissues of poultry by microbial method. Afr. J. Pharm. Pharmacol. 5(8):1034-1037.
|
|
|
|
|
Kaufmann A (2009). Validation of multiresidue methods for veterinary drug residues; related problems and possible solutions. J. Anal. Chim. Acta 637:144-155.
Crossref
|
|
|
|
|
Larkin C, Poppe C, Mcnab B, Mcewen B, Madhi A, Odumeru J (2004). Antibiotic resistance of Salmonella isolated from hog, beef, and chicken carcass samples from provincially inspected abattoirs in Ontario. J. Food Protocol. 67:448-455.
|
|
|
|
|
Loksuwan J (2002). The effect of heating on multiple residues of tetracyclines in milk. J. Sci. Technol. 7(3):17-20.
|
|
|
|
|
Mmbando LMG (2004). Investigation of oxytetracycline use and abuse: Determination of its residue in meat consumed in Dodoma and Morogoro. A thesis submitted for the award of a MVM Degree at Sokoine University of Agriculture, Morogoro, Tanzania, pp. 240.
|
|
|
|
|
Nguyen V, MuQing L, Muhammad AK, ChunBao L, GuangHong Z (2013). Effect of cooking methods on tetracycline residues in pig meat. Afr. J. Pharm. Pharmacol. 7(22):1448-1454.
Crossref
|
|
|
|
|
Nonga HE, Sungura KH, Ngowi HA (2013). Assessment of veterinary drug use and determination of antimicrobial residues in broiler chicken meat in Urban district, Zanzibar, Tanzania. Tanzania. Vet. J. 28(2):26-29.
|
|
|
|
|
Salah HA, Ali RS (2013). Effect of ordinary cooking procedure on tetracycline residues in chicken meat. J. Food and Drug Anal. 21(1):80-86.
|
|
|
|
|
Seymour H, Jones G, McGilliard M (1988). Persistence of residues in milk following antibiotic treatment of dairy cattle. J. Dairy Sci. 71:2292-2296.
Crossref
|
|
|
|
|
Shankar BP, Manjunatha BH, Chandan S (2010). Rapid methods for detection of veterinary drug residues in meat. J. Vet. World 3(5):241-246.
Crossref
|
|
|
|
|
Stolker AM, Brinkman UA (2005). Analytical strategies for residue analysis of veterinary drugs and growth-promoting agents in food-producing animals-a review. J. Chromatogr. 1067:15–53.
Crossref
|
|
|
|
|
Tajick MA, Shohreh B (2006). Detection of Antibiotics Residue in Chicken Meat Using TLC. J. Poult. Sci. 5(7):611-612.
Crossref
|
|