ABSTRACT
This study aimed to evaluate the healing and analgesic action of methanolic extract and hydroalcoholic of C. taxifolia, and check the percentage of soluble proteins available in the extract of this vegetable. The collection of C. taxifolia occurred on the beach of Barra do Sirinhaém - PE, processed for testing. The animals used in the test were Swiss mice, and Mus musculus of species. For healing activity and histopathological analysis, albino rats Rattus norvegicus species, linnhagem Wistar were used. The protein content of the crude extract of C. Taxifolia has not yet been determined. The data revealed that the average displaying writhing in animal control group was 13.86. The standard group was 7.83; the group treated with hydroethanolic extract of C. taxifolia at a dose of 50 mg/kg was 8.83 and those treated with 100 mg/kg was 6.5. In the groups treated with the methanol extract of 100 mg/kg, the average value of writhing was 7.67, while 50 mg/kg resulted in 9.26 average value. It was shown that hydroethanolic and methanol extract led to early healing ofwound with epithelialized keratinized tissue and re-epithelialization; involving the restructuring of the skin appendages, during treatment and in the absence of crust.
Key words: Healing, seaweed, protein, repair.
Wound healing is a process of repair that follows after injury of the skin and other soft tissues, and encompasses a complex series of interactions between different cell types, inflammatory mediators, and extracellular matrix. It is the phase of wound healing involving hemostasis, inflammation, proliferation and remodeling; and each phase is different, although the process is continuous (Riella et al., 2012). Since the first occurs in the first hours (hemostasis), this process occurs in platelet activation and consequent aggregation flirt and coagulation cascade. In the second stage,which occurs in days, the inflammatory process is present, and there is the recruitment of neutrophils and macrophages, which among other things help to degrade the devitalized tissue; and macrophages stimulate the growth of new tissue (Irion et al., 2005). Treatment of wound closesas quickly as possible the lesion in order to obtain a functional and aesthetically satisfactory scar. Medicinal products obtained exclusively from raw materials of vegetable origin called herbsprovide active ingredients with anti-inflammatory and wound healing applications (Michelin et al., 2005; Lima et al., 2006).
Among the products of dermatological interest, particular attention deserves the action incorporated into the topical dosage forms. This can in turn allow recovery of intact posterior skin to possible attacks. For the restoration of normal conditions, the healing process is very important. In this context, the migration of inflammatory cells in granulation tissue synthesis, deposition of collagen and proteoglycans and maturation of the scar, are associated with severe reshaping. Thus, the complementary medicine works as a tool for this treatment (Santos et al., 2002; Mendonça, 2006; Rocha et al., 2006).
Several medicines for tissue healing mostly originate in natural products (Segundo et al., 2007). These biomaterials consist of interactive elements that are able to establish adequate affinity with the surrounding tissue, without however inducing adverse response of the host (Ratner and Bryant, 2004). Among the biomaterials, polysaccharides stimulate the immune system in vitro and in vivo in order to facilitate the healing process (Diallo et al., 2001; Kweon et al., 2003; SeneL and Mcclure, 2004; Vitorino Filho, 2011).
The diversity of Brazilian marine flora favors the discovery of pharmacological agents in the prevention and treatment of diseases (Rodrigues et al., 2019). Sulphated polysaccharides (PS), and soluble proteins are found in these agents, in large concentrations and with interesting biological properties (Rodrigues et al., 2009).
These compounds have structural complexity due to the many possibilities of monosaccharides and distribution of sulfate groups, which vary from species to species, and sometimes in different parts of the plant (Alves, 2000; Haroun-Bouhedja et al., 2000).
The presence of polysaccharide sulfates can promote change in biological activity, since it modifies the conformation of its chain; makes nonpolar substances in water-soluble and also promotes interactions with cationic proteins (Shanmugam and Mody, 2000; Liu et al., 2009).
Some PS present in green algae are covalently bound to proteins, being classified as proteoglycans (Aquino et al., 2005; Ropelatto et al., 2011).
This group of organisms, which belongs to the genus
Caulerpa, appears to differ sterols on cholesterol chemical structure. The compounds are biologically important as hormones, vitamins and structural components of biomembranes (Ghosh et al., 2004; Lee et al., 2004; Shevchenko et al., 2009). Phytochemical approach to
Caulerpa taxifolia indicated the presence of secondary metabolites such as alkaloids, terpenes, sterols and saponins (Moura et al., 2012). The caulerpina isolated alkaloid
C. taxifolia was active in enterocolitis study model for mice (Lima et al., 2014). For Kathiravan et al. (2014), species of this genus can produce Ag nanoparticles and these extracellularly (nanoparticles) are quite stable in solution, likely due to leveling proteins present in the extract. This study aimed to evaluate the healing and analgesic action of methanolic extract and
hydroethanolic of
C. taxifolia, and check the percentage of soluble proteins available in the extract of this vegetable.
Collection of vegetable material
Marine macroalgae
C. taxifolia was collected on the beach of Sirinhaém Bar, county Sirinhaém - PE. Then it was placed in plastic bags with seawater and transported to the Experimental Oncology Laboratory, UFPE, where it was washed in distilled water and dried in an oven at 25°C temperature. It was later weighed and crushed in blender (Arno®) for macerating. It was put in a glass container with 50%
hidroetanóolica solution (a first portion) and another portionof methanolic solution of 100%. After 7 days of soaking the material was placed in rotatory evaporator and stored until the day of the test. The performance of hydroethanolic and methanol extract were 11.5 and 8.8% respectively.
Laboratory animals
The animals used in the test of writhings were Swiss mice of the Mus musculus species; they were males of approximately 60 days old. They weighed 25 to 30 g after birth. They were kept in light controlled conditions (12-hour light / dark cycle) and temperature of 22 ± 2°C in polypropylene cages, where they received specific food and water ad libitum. 12 h prior to the study, the animals were fasted.
For the activity of healing, the animals used were the albino rats of the species Rattus norvegicus, linnhagem Wistar, of 120 days; weighing between 180-200 g. They were kept in controlled lighting conditions (12 h light / dark cycle) and temperature of 22 ± 2°C in polypropylene cages, where they received specific food and water
ad libitum. 12 h before the surgical procedures, the animals were fasted. The present study was approved by the research ethics committee in animals of the Federal University of Pernambuco, being registered under the protocol number 179/04.
Nociceptive activity
The writhing test in mice was carried out according to Koster et al. (1959). The methanolic and hydroethanolic
extracts of
C. taxifolia were solubilized in dimethyl sulfoxide: Tween 80 (1: 1) 1% (v / v) in saline and administered orally 1 hour before the application of0 6% acetic acid. The control group (group 1) received 0.3 ml / 30 g of dimethyl sulfoxide: Tween 80 (1: 1) 1% in saline orally (po). Indomethacin (10 mg/kg) was the reference drug administered orally to mice in the positive control group (group 2). Groups 3 and 4 were administered doses of 50 and 100 mg/kg of hydroethanolic extract of
C. taxifolia respectively; 50 and 100 mg/kg of methanolic extract of
C. taxifolia were given to Groups 5 and 6, respectively; rat weight (n=10). One hour after treatment, 10 ml/kg of 0.6% acetic acid was administered intraperitoneally in each mouse and the number of writhings was counted within a range of 10 and 30 min after this procedure.
The percentage inhibition of writhing was calculated by the formula:% Inhibition = [(NC control - NC treaty) ÷ NC control] × 100 where: NC control: number of writhes in the control group; NC treaty: writhing number of the treated group.
Getting cream
The cream for the treatment of animals used in the healing experiment was obtained by adding extracts and solubilization of the cream base, in GLOBO® manipulated manipulation pharmacy. Constituents of base cream are: Water, 34.5%; Glycerin, 6.0, 5.0% cetostearyl alcohol; 3.0% glyceryl monostearate; sodium lauryl sulfate ester, 1.5% (Batista et al., 2011).
Healing test
For the healing test 60 animals were selected which were divided into 12 groups (n = 5), where three groups each were treated topically with methanolic extract of
C. taxifolia; hydroethanolic extract of
C. taxifolia, cream base, and
Bepantol cream. During the treatment there was removal of scar tissue of one of the three groups at 7, 14 and 21 days.
Histopathological evaluation
All samples were fixed in 10% formalin, embedded in paraffin, cut in 5 mm and stained with hematoxylin-eosin (HE). These sections were then examined under a light microscope to detect histological changes
by a histopathologist who did not know the groups. Slides were scored for the presence of vascularisation, edema, and degrees of acute and chronic inflammation.
Morphological analysis
The morphometric analysis was performed on histological sections stained by HE. Each slide was measured by a high-power field magnified 400x including the healing of the incision area; the average number of collagen bundles in each group was calculated.
Protein soluble analysis
The Bradford method (1976) was employed to determine the protein content of the crude extract of C. taxifolia. The reagent was prepared by solubilizing 50 mg Coomassie in 25 mL of ethanol (95%), with further addition of 100 ml of phosphoric acid (85% w/v). The final concentrations (w/v) of the reagent was 0.01% Coomassie, 4.7% ethanol and 8.5% phosphoric acid. Standard albumin solutions were prepared in increasing concentrations of 1.0 to 0.1 mg/mL, from dilutions of the stock solution (5 mg/ml).
Vegetable processed samples were cut into pieces (5 mm) and 200 mg was weighed and homogenized in 10 ml of 80% ethanol. An aliquot of the crude extract was centrifuged for 5 min at 2,000 g; an aliquot of 3 ml of the supernatant was transferred to a test tube, and 6 ml of chloroform wasadded to it.This mixture was stirredcontinously for two minutes gently and then left unstirred for 10 min. So, there were two phases (organic and aqueous). The aqueous fraction (colorless) was collected in an eppendorf and kept in freezer until the time of color development.
For color development, pipetted 200 uL of standard solutions and sample extracts were added to 4 mL of the Coomassie brilliant blue reagent. The tubes were subjected to gentle agitation for 5 min at rest. Then the readings were performed in a spectrophotometer at a wavelength of 595 nm; the amounts of soluble protein content were expressed in terms of mg of soluble protein per gram of fresh plant tissue (Bradford, 1976).
The concentration of soluble proteins was calculated by the formula: Focuses = [(White Abs - Abs sample) ÷ white Abs] × 100 where: Abs blank: absorbance measured for white; Abs sample: absorbance measured for the samples.
Statistical analysis
The test of writhing was considered significant for values ​​*** p < 0.001, after analysis of variance (ANOVA) followed by Student Newman -Keuls test when compared to the control group.
The average displaying writhing in animal control group was 13.86; the standard group average was 7.83. The group treated with hydroethanolic extract of C. taxifolia at a dose of 50 mg/kg had 8.83; the group treated with hydroethanolic extract of C. taxifolia at a dose of 100 mg/kg showed an average value of 6.5. The groups treated with methanolic extract of C. taxifolia at a dose of 100 mg/kg had an average value of 7.67 and the twisted group treated with methanolic extract of seaweed at a dose of 50 mg/kg had 9.26 contortions as mean value (Table 1).
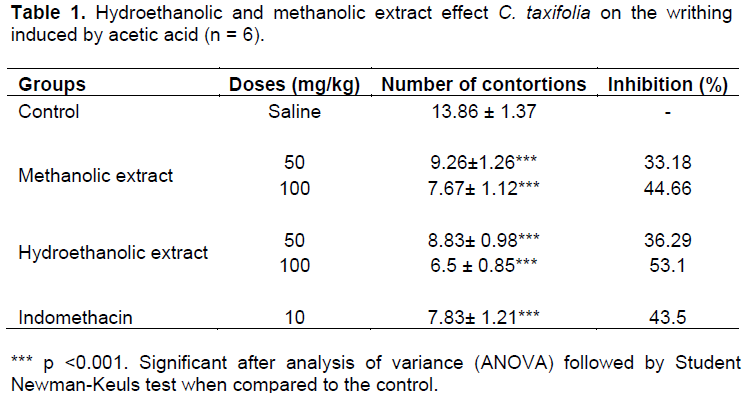
The control group treated with 50 mg/kg hydroethanolic extract had 36.3% decrease in writhing. T100 mg/kg hydroethanolic extract reduced writhing by 53.1%. With the methanolic extract of 50 and 100 mg/kg, there was a reduction of 33.2 and 44.7% respectively.
Soluble proteins
In the spectrophotometric quantification of octoplicata-samples as soluble protein present in extract of C. taxifolia, readings were done according to the formula described by Bradford (1976), which was expressed as the mean ± standard error of the mean. The result was 6.67 ± 1.32 µg /mg.
Healing activity
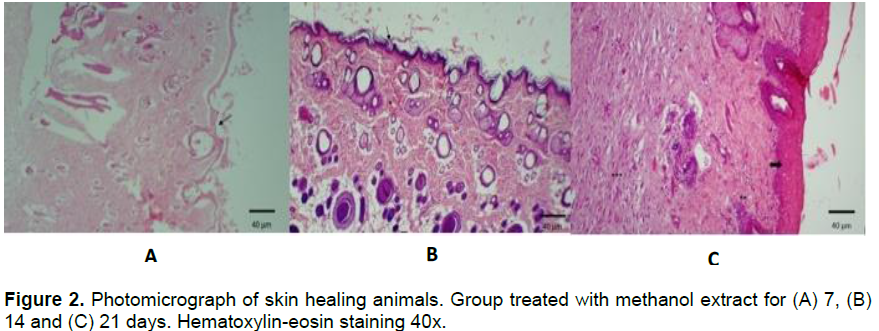
It was shown that both the inorganic and organic extracts showed early wound healing with epithelialized keratinized tissue; with restructuring of the skin appendages, during the treatment and in the absence of crust (Figures 1 and 2). However, the animals treated with the organic extract (21 days) showed inflammatory infiltrates (**) and a significant number of fibroblasts (***) when compared to treatments of 7 and 14 days.
The data demonstrate that both extracts reduced writhing in mice with two doses tested, suggesting inhibition of prostaglandin synthesis via cyclooxygenase, as well as indomethacin. Positive control test has the mechanism of inhibiting this enzyme (Duarte et al., 1992). The inhibition of contortions were quite significant at the tested doses, most apparent with the hydroethanolic extract of 100 mg/kg.
The test of writhings is a chemical model of nociception that is based on counting the contortions of the abdominal wall followed by the trunk torsion, and extension of the hind limbs, such as reflex response to peritoneal irritation and peritonitis produced by intraperitoneal injection of a solution of 0.9% acetic acid (
Whittle, 1964). This test is sensitive to the evaluation of analgesic drugs; however, it can be seen as a general model, non-selective, for studies of antinociceptive drugs (Couto et al., 2011).
Para Julius and Basbaum (2001) sprotons coming from the dissociation of acetic acid can directly activate the nonselective cation channels located in primary afferent pathways. The local irritation produced by intraperitoneal injection of acetic acid causes the release of a variety of mediators such as substance P, bradykinin, prostaglandins, as well as the pro-inflammatory cytokines such as IL-1, IL-6, IL-8 and TNF-α (Pinheiro et al., 2011). Verma et al. (2005) comment that this method is associated with the release of prostanoids, in general, high levels of PGE2 and PGF2a and lipoxygenase products in peritoneal fluid.
The extracts of
C. taxifolia (hydroethanolic and m
ethanol) exhibited antinociceptive activity in the model induced by acetic acid. The responses were significant at both doses of extract, and the percentage of inhibition increased with increasing dose. This can be related with the synthesis of prostaglandins via cyclooxygenase as well as indomethacin (Sousa, et al., 2009).
The substance P and bradykinin are involved in the first phase, while histamine, serotonin, prostaglandins and bradykinin participate in the second phase of the response (Shibata et al.,1989). Morrow and Roberts II (2001) and Costa-and-Sousa (2010) indicate that the test of writhings involves anti-inflammatory mechanisms. In the process of tissue repair, under physiological conditions, fibroblasts are encouraged to migrate to the area of injury and produce collagen fibers to effect this process (Honorio-França et al., 2008; Mendonça; Coutinho Netto, 2009; Dias, 2012). In skin lesions, it is possible to assess the stage of tissue damage by histological analysis (qualitative and quantitative) of the main features that show the evolution of this process, such as the number of inflammatory cells, fibroblasts, and of new blood vessels formed by angiogenesis process (Batista et al., 2010; Oliveira et al., 2010).
Moreover, macrophages stimulate the growth of new tissue, as observed in animals in the 7th day, as well as the presence of small collagen fibers. The third stage of healing that occurs around days to weeks, was more intensified and there was continuous reepithelization of the formation of granulation tissue. This fact can be seen in animals related to the 14th day. The presence of macrophages at this stage stimulates production of fibroblasts and deposition of loose connective tissue; whereas the collagen produces fibroblast migration. It was noticed that in the group of animals on the 21st day, the third stage was still present. This is explained by the depth of the lesion, which can be a limiting factor for the evolution of healing (Irion, 2005).
The extracts appear to have acted in the healing of treated animals promoting epithelization. According to Modolin and Bevilacqua (1985), at the end of the proliferative phase is re-epithelization of the lesion, which is controlled by chalona, a glycoprotein complex that stimulates epithelial mitotic activity.
Rubin and Farber (2002), Stevens and Lowe (2002) say proliferative phase lasts for 12 to 14 days, and is characterized by repairing the connective tissue with granulation tissue formation and consecutive repitelização. The repair process begins with inflammation and around 24 h after injury there is no resolution; fibroblasts and vascular endothelial cells initiate proliferation forming scar tissue, that is, granulation. It is histologically characterized as vasculogenesis and has increased numbers of fibroblasts.
Agnol (2008) further notes that in the repair process, by the second and third day after injury, the collagen-producing fibroblasts are recruited from the shores of injury and induce protein synthesis by fibroplasia. Consequently, the fibrinogen present in the inflammatory exudate turns into fibrin, which will serve for adhesion and proliferation of fibroblasts, which secrete scar tissue.
Therefore,
the silver nanoparticles found in Caulerpa taxifolia possibly leveraging (in conjunction with) their potential antibiotics may be responsible for the healing effect since, with the control of the local microbiota developed, the mechanism for healing does not sufferinterference.
The authors have not declared any conflict of interest.
REFERENCES
|
Alves LG, (2000). Polissacarídeo acidos presentes no folíolo, talo e flutuador da alga marinha sargassum vulgare. Dissertação (Programa de Pós-graduação em Bioquímica) – Departamento de Bioquímica – Universidade Federal do Rio Grande do Norte- UFRN, NATAL.
|
|
|
|
Aquino RS, Landeira-Fernandez AM, Valente AP, Andrade LR, Mourão PAS (2005). Occurrence of sulfated galactans in marine angiosperms: evolutionary implicatios. Glycobiology 15:11-20.
Crossref
|
|
|
|
|
Batista JS, OlindaI RG, MedeirosI VB, Rodrigues CMF, Oliveira AF, Paiva ES, Freitas CIA, Medeiros AC (2011). Atividade antibacteriana e cicatrizante do óleo de buriti Mauritia flexuosa L. Ciencia Rural, Santa Maria.
|
|
|
|
|
Bradford MM (1976) A rapid and sensitive method for the qualification of microgram quantities of protein utlizy the principle of protein dye binding. Anal. Biochem. 7:248-254.
Crossref
|
|
|
|
|
Couto VM, Vilela FC, Dias DF, Santos MH, Soncini R, Nascimento CG, Giusti-Paiva A (2011). Antinociceptive effect of extract of Emilia sonchifolia in mice. J. Ethnopharmacol. 134(2):348-353.
Crossref
|
|
|
|
|
Diallo D, Paulsen BS, Liljebäck TH, Michaelsen TE (2001). Polysaccharides from the roots of Entata africana Guill. Et Perr Mimosaceae, with complement fixing activity. J. Ethnopharmacol. 74:159-171.
Crossref
|
|
|
|
|
Duarte IDG, Ferreira-Alves DL, Nakamura-Craig M (1992). Possible participation of endogenous opioid peptides on the mechanism involved in analgesia by vouacapan. Life. Sci. 50:891-897.
Crossref
|
|
|
|
|
Haroun-Bouhedja F, Mostafa E, Sinquin C, Boisson-Vidal C, (2000). Relation between sulfate group and biological activities of fucans. Thromb. Res. 100:453-459.
Crossref
|
|
|
|
|
Julius D, Basbaum AI (2001). Molecular mechanisms of nociception. Nature 143:203-210.
Crossref
|
|
|
|
|
Koster R, Anderson M, Beer EJ (1959). Acetic acid for analgesic screening. Fed. Proceed. 18:412-21.
|
|
|
|
|
Kweon DK, Song SB, Park YY (2003). Preparation of water-soluble chitosan/heparin complex and application as wound healing accelerator. Biomaterials 24(9):1595-601.
Crossref
|
|
|
|
|
Liu Y, Liu C, Tan H, Zhao T, Cao J, Wang F (2009). Sulfation of a polysaccharideo obtained from phellinus ribis and potencial biological activities of the sulfated derivatives. Carbohydrate Polymers 77:370-375.
Crossref
|
|
|
|
|
Modolin M, Bevilacqua RG (1985). Cicatrização de feridas. Síntese das aquisições recentes. Rev. Bras. Clín. Terap 14:208-213.
|
|
|
|
|
Pinheiro B, Silva A, Souza G, Figueiredo J, Cunha F, Lahlou S, Da Silva JK, Maia JG, Sousa PJ (2011). Chemical composition, antinociceptive and anti-inflammatory effects in rodents of the essential oil of Peperomia serpens (Sw.) Loud. J. Ethnopharmacol. 188:479-486.
Crossref
|
|
|
|
|
Rodrigues JAG, Souza Junior J, Lourenço JÁ, Lima PCWC, Farias WRL (2009) Cultivation of shrimps treated with sulfated polysaccharides of Halymenia pseudofloresia rhodophyceae through a prophylactic strategy. Rev. Cienc. Agron. 40(1):71-78.
|
|
|
|
|
Ropelatto J (2011). Estrutura química e atividade antitumoral de heteroramnanas sulfatadas e seus produtos de degradação parcial obtidos da macroalga verde Gayralia oxysperma. UFPA, Curitiba.
|
|
|
|
|
Segundo SA, Maia DD, Ribeiro RV, Aguiar EHD, Rocatto GEGD, Cirilo DM, Samyra LB, Semenoff TADV (2007). Influência do Aloe vera e própolis na contração de feridas em dorso de ratos. Periodontia 17(1):23-28.
|
|
|
|
|
Senel S, McClure SJ (2004). Potencial applications of chitosan in veterinary medicine. Adv. Drug Deliv. Rev. 56(10):1467-1480.
Crossref
|
|
|
|
|
Shanmugam M, Mody KH (2000). Heparinoid-active sulphated sulphated polysaccharides from marine algae as potential blood anticoagulant agents. Curr. Sci. 79(12):1672-1683.
|
|
|
|
|
Sousa OV, Fioravante IA, Del-Vechio-Vieira G, Caneschi CA, (2009). Ivestigação das atividades antinociceptiva e antiedematogenica do extrato etanólico das folhas de Joannesia princeps Vellozo. Rev. Ciênc. Farm. Básica Appl. 30(1):91-97.
|
|
|
|
|
Verma PR, Joharapurkar AA, Chatpalliwar VA, Asnani A. (2005). Antinociceptive activity of alcoholic extract of Hemidesmus indicus R. Br. in mice. J. Ethnopharmacol. 102:298-301.
Crossref
|
|
|
|
|
Vitorino-Filho RNL (2011). Uso de polissacarídeo extraído do exsudato de cajueiro (Anacardium occidentale L.) na terapêutica tópica de feridas (Doctoral dissertation, Dissertação (Mestrado em Ciência Animal)–Universidade Federal do Piauí, Teresina).
|
|
|
|
|
Whittle BA (1964). Release of a kinin by intraperitoneal injection of chemical agents in mice. J. Neuropharmacol. 3:369-378.
Crossref
|
|