ABSTRACT
Gas chromatography-mass spectrometry (GC-MS) analysis and antioxidants activity of phytochemical constituents from ethyl acetate fraction of Vernonia calvoana (V.C) was evaluated. The fraction was subjected to GC–MS analysis using 5% diphenyl 95% dimethylpolysiloxane (30 m × 0.25 mm ID × 0.25 µm film thickness), the compounds were separated and identified by Turbo gold mass detector. The antioxidants activity was investigated employing the 1, 1-diphenyl-2-picrylhydrazyl radical (DPPH) and Ferric reducing antioxidant power (FRAP) scavenging method. Eighteen different phytochemical compounds namely: Etylobenzene (22.13%), isopropylidenecyclopentadiene (8.24%), 1-ethyl-2-methylbenzene (8.64%), decane (15.24%), 1,2,3-trimethylbenzene (12.51%), 2,7-Dimethyloctane (5.79%), 4-methyltridecane (0.72%), tetrahydro-2-furanmethanol (2.44%), 3,3-Dimethylhexane (0.54%), 5-methylundecane (0.74%), 1-Nonyne (2.16%), 1-pentadecane carboxylic acid (4.70%), 2,6-Dimethyl-1,7-octadien-3-ol (4.00%), cis-9-octadecanoic acid (5.49%), n-Nonadecanoic acid (1.53%), Nonyl propyl ester (1.84%), 5,8-octadecadienoic acid (1.79%), and 9,12,15-octadatrienal (1.53%) were identified. Increase in activity was observed in both the DPPH and FRAP analysis, and were concentrations dependent and closely related to ascorbic acid. The phytochemical components using GC-MS and antioxidant activity of ethyl acetate extract of V.C have been evaluated. In addition to this, further isolation and characterization of individual components would provide more insight to its use in the production of new drugs of therapeutic value.
Key words: 1, 1-diphenyl-2-picrylhydrazyl radical (DPPH), Ferric reducing antioxidant power (FRAP), gas chromatography, Vernonia calvoana, antioxidants.
Before the advent of synthetic drugs, man depended on the curative properties of medicinal plants. The value for the use of these plants originate from ancient belief which says God created plants to provide food, medical treatment, and other effects for man (Ahvazi et al., 2012). Medicinal plant serves as the first line for primary health care to the rural populace and “backbone” of traditional medicine; this suffice that, more than 3.3 billion people in the less developed countries make use of medicinal plants almost on a regular basis for curative purposes (Davidson-Hunt, 2000). Vernonia (Asteraceae) is the largest genus in the tribe Vernoniae, with over 1000 species (Keeley and Jones, 1979). The genus is known for having several species with both nutritional (Igile et al., 1995) and industrial importance (Perdue et al., 1986). The genus, overtime, have been popularly used in the management of different disorders including inflammation, malaria, fever, worms, pain, diuresis, cancer, abortion, and several gastrointestinal problems (Alves and Neves 2003).
Also based on pharmacological use, Vernonia species have been documented to possess hypotensive activity (Achola et al., 1996), phototoxic, antibacterial and anti-inflammatory (Perez-Amador et al., 2008), immunomodulatory (Nergard et al., 2004), antioxidants (da Silva et al., 2013) and anti-histaminic activity (Laekeman et al., 1983). These activities reported may be attributed to diverse secondary metabolites such as flavonoids, anthraquinones, polyphenols, tannins, alkaloids, sesquiterpene lactones, phenol, steroidal saponins, cardiac glycosides, carotenoids and high nutritive components present in this species (Ejoh et al., 2005; Igile et al., 2013). Vernonia calvaona Hook F (Astereaceae) is popularly called “Ekeke leaf” by the people of Ugep in Yakurr Local Government Area of Cross River State of Nigeria. It is a small plant of about 1 m in height and leaves of 10.0 mm wide. It is eaten raw with pepper sauce, leaving a sweet taste in the tongue. It is also used to prepare native soups (melon soup) in place of its sister plant Vernonia amygdalina to provide slight bitter taste.
Documented reports have shown that extracts from this plant possess hypoglycemic, hypolipidemic, and in vivo antioxidant activity in diabetic animal which could make available the needed information to choose the extract from this plant for further biochemical investigations (Iwara et al., 2015 and 2017). Also report by Egbung et al. (2016) showed that the inflorescents from the plant possess a strong antioxidant activity compared to ascorbic acid. Antioxidants are compounds that have the potentials of delaying or inhibiting the oxidation of other molecules by blocking the oxidizing chain reactions. The molecule have the ability of neutralizing free radical by donating electrons and further prevent damages that may be caused by this radical to cell and tissue (da Silva et al., 2013). Fruits, vegetables and herbs are good sources of these compounds which are known as phytochemicals including flavonoids, ascorbic acids etc. These phytochemicals are documented to have corresponding characteristics with antioxidant potentials (Katalinic et al., 2004). In this study, the phytochemical components of V. calvoana would be evaluated using GC-MS with further investigation of its in-vitro anti-oxidant activity using Ferric reducing antioxidant power (FRAP) and 1, 1-diphenyl-2-picrylhydrazyl radical (DPPH) scavenging method.
Sample collection and preparation
Fresh leaves of V. calvoana were harvested from a farm in Ugep, in Yakurr L.G.A of Cross River State, Nigeria. The leaves were collected in the early hours of the day, cleaned and air dried for 7 days after which they were ground into powder form. A measured quantity of 5 kg of powder leaves were extracted via cool maceration in 8 L of 80% ethanol for 48 h. The extract was further double filtered with chess cloth, then with filtered paper (Whatman 4 filtered paper) and the residue obtained was further extracted with 4 L of 80% ethanol. The filtrate was then concentrated at 45°C in rotary evaporator (RE-52A, Shangai Ya Rong Biochemistry Instrument Company,China) to 10% volume and then to complete dryness using water bath yielding 310.3 g (6.2%) of crude extract. The obtained crude extract was subjected to fractionation.
Fractionation of crude extract
The crude extract (251.8 g) was chromatographically eluted with ethyl acetate in a column packed with silica gel of mesh 60 to 120. The fractions were collected and evaporated in rotary evaporator at 50°C to 10% of its original volume and further evaporated to paste form in a water bath at 50°C. The percentage yield for the fraction was 20 g (8.10%) ethyl acetate fraction. The fraction and the remaining crude extract were stored in a freezer at -4°C for further experiments.
Gas chromatography–mass spectrum (GC-MS) analysis
Analysis of fractionated samples was carried out using GC-MS -QP2010 plus (SHIMADZU-JAPAN), comprising of AOC-20i auto sampler and gas chromatograph interfaced with a mass spectrometer. The assay conditions were as follows: fused silica capillary column (Rastek RT × 5Ms; 30 m × 0.25 mm ID × 0.25 um film thickness) composing of 5% diphenyl 95% dimethylpolysiloxane; column oven temperature at 80°C, injection temperature maintained at 250°C; injection mode split; pressure of 108 kpa; total flow of 6.2 ml/min at 1 ml/min; column flow of 1.58 ml/min; split ratio of 1.0 and solvent cut time of 2.50 min. Mass spectra were taken at start time of 3.0 min and end time of 27.0 min; while the ACQ mode- Scan was carried out at event time (0.50 s), with scan speed 1250 m/s.
Identification of component
Identification of components on the chromatogram was carried out using the database of National Institute Standard and Technology (NSIT) containing over 62,000 patterns. The spectrum of the unknown sample was compared with that of the known components present in the library. Molecular weight, name and the structural formula of the unknown sample were then ascertained.
In vitro antioxidant estimation
Estimation of antioxidant capacity using the 1, 1-diphenyl-2-picrylhydrazyl radical (DPPH) technique
Standard method according to Mensor et al. (2001) was employed in elucidation of scavenging activity of extracts of Vernonia calvoana. Two mils of the extract at different concentration ranges 10 to 400 µg/ml was mixed with 1 ml of 0.5 mM DPPH (in methanol) in a cuvette. The absorbance was measured at 517 nm after 30 min incubation for 30 min in the dark at room temperature. Methanol (1.0 ml) mixed with 2.0 ml of the sample was used as blank while ascorbic acid was used as standard. The percentage antioxidant activity was calculated using the formula below:
% Antioxidant Activity [AA] = 100 - [{(Abs sample - Abs blank) × 100}/Abs control]
Ferric reducing antioxidant power (FRAP) assay
The scavenging capacities of extract were elucidated using FRAP assay method according to Benzie and Strain (1999) with modification by Udeh et al. (2011). “FRAP assay determines the change in blue colored FeII-tripyridyltriazine compound at absorbance of 593 nm from a colorless oxidized FeIII by the reaction of antioxidants. Standard curve was prepared using different concentrations (100 to 1000 µmol/L) of FeSO4 x 7H2O. Solution (working solution) for the assay was freshly prepared by mixing 2 ml acetate buffer, 2.5 ml TPTZ and 2.5 ml FeCl3. 6H2O. The temperature of the solution was 37°C before use. In the assay, the antioxidant capacity of the extracts was calculated with reference to the reaction signal given by a Fe2+ solution of known concentration. Ascorbic acid was measured within 30 min after preparation, and calculations were made using the formula.
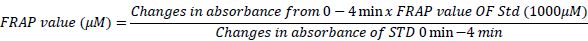
Statistical analysis
The statistical analysis was carried out using SPSS (21.0) and results were presented as mean ± SD. The degree of freedom was determined using student t-test and values of P<0.05 are considered significant.
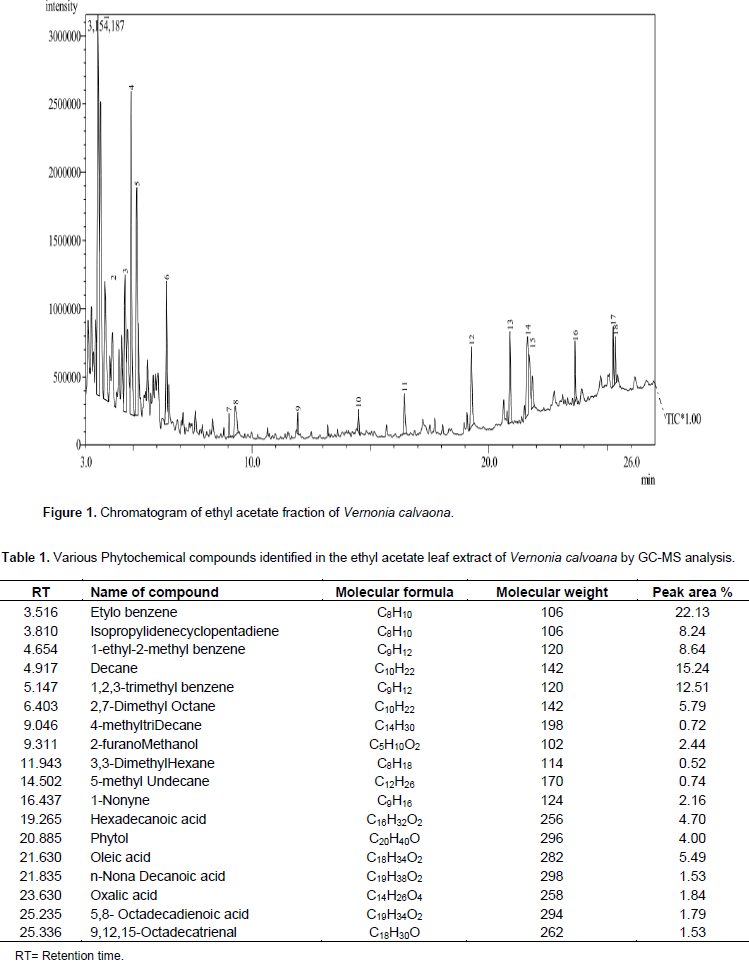
The results of the phytochemical screening of the ethyl acetate fraction of
V. calvoana leaves is presented in Figure 1 and Table 1 present the chromatogram and identified compounds of ethyl acetate of
V. calvoana, their names, percentage peak area, molecular weight, retention time, and molecular formula. The results indicated the presence of eighteen compounds in ethyl acetate fraction of
V. calvoana leaves. Etylobenzene was observed to be highest present, followed by decane, 1,2,3-trimethylbenzene,1-ethyl-2-methylbenzene and isopropylidenecyclopentadiene, other compounds where found to be present in trace concentrations. Oleic (linolenic acid -5.69%) acid and hexadecanoic acid (palmitic acid -4.70%) have been reported to have hypocholesterolemic, antioxidant and lubricating activity (Selvamangai and Bhaska, 2012). Oleic acid is commonly found in diet. It is a
monounsaturated fat which on consumption has been linked with decreased
low-density lipoprotein (LDL) cholesterol, and possibly increased
high-density lipoprotein (HDL) cholesterol (Teres et al., 2008). Palmitaldehyde which is a derivative of palmitic acid has been reported to function in the stabilization of human erythrocyte membrane (Zavodnik et al., 1996).
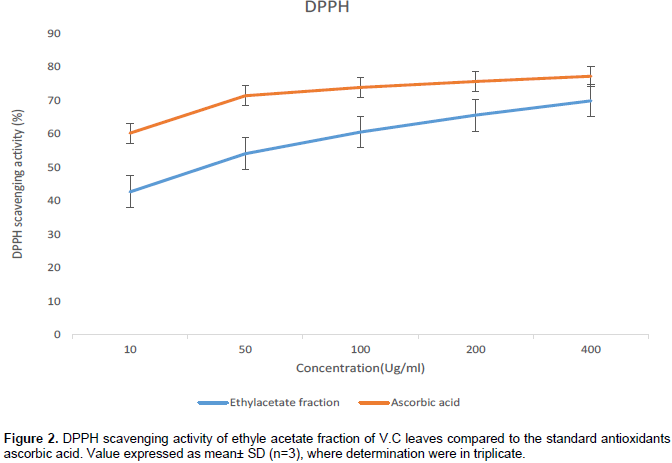
Assay for free radical scavenging activity falls among the most widely used methods in establishing activity of medicinal plant extract and their bioactive components. DPPH has the ability to remove labile hydrogen, and the capacity to scavenge the DPPH radical correlates the inhibition of lipid peroxidation (Matsubara et al., 1999). DPPH radical has odd electron, which is responsible for the deep purple coloration observed when it accepts an electron donated by an antioxidant. From Figure 2, it was observed that, the extract inhibited DPPH activity in a dose-dependent manner, with significant potency observed in 400 ug/ml concentration which compared closely to ascorbic acid. Data from the study clearly shows that V.C leaves has high electron/hydrogen-donating secondary metabolites and may function as active antioxidants; “Least amount of activity in the least polar extract and highest amount of activity in the most polar extract” (Atangwho et al., 2013).
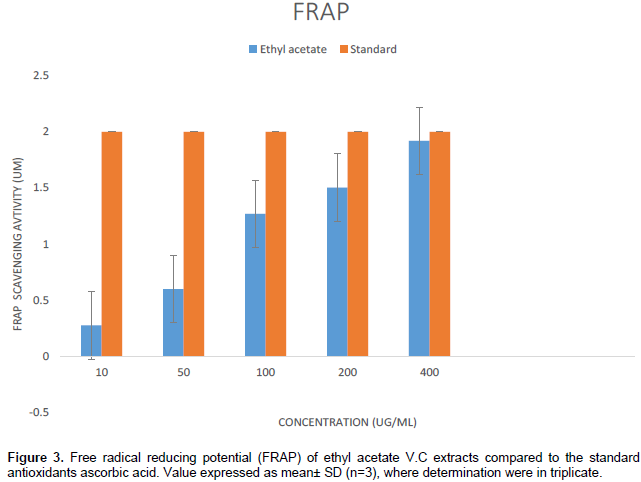
Although, ethyl acetate is known to be a moderately polar solvent, relatively non-toxic and volatile chemical, it was noted that the antioxidant activity was dose dependent which may further be attributed to the polarity of the extraction solvent. The observation is in agreement with the report of Oboh et al. (2008), which showed that the polar extracts of leafy vegetables are better DPPH scavengers than the non-polar extract. The ability of V. calvoana extract to reduce ferric ions was estimated using FRAP assay (Figure 3). The change in color was from colorless oxidized Fe+3 in absorbance at 593 nm owing to the formation of blue colored Fe+2- tripyridyltriiazine (TPTZ) by the action of electron donating antioxidant. The FRAP values of extract was found to be higher as compared to ascorbic acid (standards) (Figure 3). This observed activity was dose dependent with significant (P<0.05) activity observed at does level of 400 ug/ml. Findings from this study is consistent with earlier report by Egbung et al. (2016), indicating that the inflorescents from V. calvoana possess a high antioxidant activity that is comparable to that of ascorbic acid.
In this study, the phytochemical constituents present in ethyl acetate fraction of V. calvoana and their ability to function as an antioxidant in neutralizing DPPH and FRAP, producing free radical into its non-reactive forms was investigated . Data from the study clearly shows that V. calvoana leaves has high electron/hydrogen-donating secondary metabolites and may function as active antioxidants. Thus, these findings further suggest that this plant is a potential source of natural antioxidant that could have great importance as therapeutic agents in preventing or slowing down oxidative stress related degenerative diseases, such as cancer and various other human ailments. However, more studies are needed for the isolation and characterization of this active biocomponents and also further biochemical studies are needed for understanding their possible mechanism of action as better antioxidants.
The authors have not declared any conflict of interests.
REFERENCES
|
Achola KJ, Mwangi JW, Munenge RW, Mwaura AM (1996). Pharmacological activities of Vernonia glabra. Int. J. Pharmacogn. 34(2):141-144.
Crossref
|
|
|
|
Ahvazi M, Khalighi-Sigaroodi F, Charkhchiyan MM, Mojab F, Mozaffarian VA, Zakeri H (2012). Introduction of medicinal plants species with the most traditional usage in Alamut region. Iran. J. Pharm. Res.: IJPR, 11(1):185.
|
|
|
|
|
Alves VFG, Neves LJ (2003). Anatomia foliar de Vernonia polyanthes Less (Asteraceae), Revista da Universidade Rural do Rio de Janeiro, Serie Ci ´ encias da ViDa, 22(2):1-8.
|
|
|
|
|
Atangwho IJ, Egbung GE, Ahmad A, Yam MF, Smawi MZ (2013). Antioxidant versus anti-diabetic properties of leaves from Vernonia Amygdalina Del. growing in Malaysia. Food Chem. 141(4):3428-3434.
Crossref
|
|
|
|
|
Benzie IFF, Strain JJ (1996). The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal. Biochem. 239(1):70-76.
Crossref
|
|
|
|
|
da Silva JB, Temponi VDS, Gasparetto CM, Fabri RL, Aragão DMDO, Pinto NDCC, Ribeiro A, Scio E, Del- Vechio-Viera G, Sousa OVDS, Alves MS (2013). Vernonia condensata Baker (Asteraceae): A promising source of antioxidants. Oxid. Med. Cell. Longev. 2013.
Crossref
|
|
|
|
|
Davidson-Hunt I (2000). Ecological ethnobotany: Stumbling toward new practices and paradigms. MASA J.16:1-13.
|
|
|
|
|
Egbung GE, Atangwho IJ, Kiasira ZB, Iwara IA, Igile GO (2016). Antioxidant activity of the inflorescents of Vernonia calvoana growing in Yakurr Local Government Area of Cross River State, Nigeria. Glob.J. Pure Appl. Sci. 22(2):141.
Crossref
|
|
|
|
|
Ejoh AR, Tanya AN, Djuikwo NA, Mbofung CM (2005). Effect of processing and preservation methods on vitamin C and total carotenoid levels of some Vernonia (bitter leaf) species. Afr. J. Food Agric. Nutr. Dev. 5(2):105-117.
|
|
|
|
|
Igile GO, Iwara IA, Mgbeje BIA, Uboh FE, Ebong PE (2013). Phytochemical, Proximate and Nutrient Composition of Vernonia calvaona Hook (Asterecea): A green-leafy vegetable in Nigeria. J. Food Res. 2(6):1.
Crossref
|
|
|
|
|
Igile GO, Oleszek W, Burda S, Jurzysta M (1995). Nutritional Assessment of Vernonia amygdalina Leaves in Growing Mice. J. Agric. Food Chem. 43(8):2162-2166.
Crossref
|
|
|
|
|
Iwara IA, Igile GO, Uboh FE, Eyong EU, Ebong PE (2015). Hypoglycemic and hypolipidimic potentials of extract of Vernonia Calvoana on alloxan-induced diabetic albino wistar rat. Euro. J. Med. Plant. 8(2):78-86.
Crossref
|
|
|
|
|
Iwara IA, Igile GO, Uboh FE, Eyong EU, Elot KN, Eteng MU (2017). Biochemical and antioxidants activity of crude, methanol and n-hexane fractions of Vernonia calvoana on streptozotocin induced diabetic rat. J. Pharmacogn. Phytother. 9(3):24-34.
Crossref
|
|
|
|
|
Katalinic V, Milos M, Modun D, Music I, Boban M (2004). Antioxidant effectiveness of selected wines in comparison with (+)-catechin. Food Chem. 86(4):593-600.
Crossref
|
|
|
|
|
Keeley SC, Jones SB (1979). Distribution of pollen types in Vernonia (Vernonieae: Compositae). Syst. Bot. 4:195-202.
Crossref
|
|
|
|
|
Laekeman GM, Mertens J, Totte J, Bult H, Vlietinck AJ, Herman AG (1983). Isolation and pharmacological characterization of vernolepin. J. Nat. Prod. 46(2):161-169.
Crossref
|
|
|
|
|
Matsubara N, Fuchimoto S, Iwagaki H, Nonaka Y, Kimura T, Kashino H, Edamatsu R, Hiramatsu M, Orita K (1999). The possible involvement of free radical scavenging properties in the action of cytokines. Res. Commun. Chem. Pathol. Pharmacol. 71(2):239-242.
|
|
|
|
|
Mensor LL, Meneze FS, Leita GG, Reis AS, do Santos TC, Coube SC, Leita SG (2001). Screening of Brazilian plants extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 15(2):127-130.
Crossref
|
|
|
|
|
Nergard CS, Diallo D, Michaelsen TE, Malterud KE, Kiyohara H, Matsumoto T, Yamada H, Paulsen BS (2004). Isolation, partial characterisation and immunomodulating activities of polysaccharides from Vernonia kotschyana Sch. Bip. ex Walp. J. Ethnopharmacol. 91(1):141-152.
Crossref
|
|
|
|
|
Oboh G, Raddatz H, Helen T (2008). Antioxidant properties of polar and nonpolar extracts of some tropical green leafy vegetables. J. Sci. Food Agric. 88(14):2486-2492.
Crossref
|
|
|
|
|
Perdue RE, Carlson KD, Gilbert MG (1986). Vernonia galamensis, potential new crop source of epoxy acid. Econ. Bot. 40(1):54-68.
Crossref
|
|
|
|
|
Perez-Amador MC, Ocotero VM, Benitez SP, Jimenez FG (2008). Vernonia patens Kunth, an Asteraceae species with phototoxic and pharmacological activity, Phyton (Buenos Aires), 77:275-282.
|
|
|
|
|
Selvamangai G, Bhaskar A (2012). GC-MS analysis of Phytocomponent in the methanolic extract of Eupatorium triplinerve. Asian Pac. J. Trop. Biomed. 2(3):S1329-S1332.
Crossref
|
|
|
|
|
Terés S, Barcelo-Coblijn G, Benet M, Alvarez R, Bressani R, Halver JE, Escribá PV (2008). Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Proc. Natl. Acad. Sci. 105(37):13811-13816.
Crossref
|
|
|
|
|
Udeh NE, Nwachujor CO (2011). Antioxidant and hepatoprotective activities of ethyle acetate leaf extract of icacina trichantha on paracetamol- induced liver damage in rats. Cont. J Anim. Vet. Res. 3:11-75.
|
|
|
|
|
Zavodnik IB, Lapshina EA, Palecz D, Bryszewska M (1996). The effects of palmitate on human erythrocyte membrane potential and osmotic stability. Scand. J. Clin. Lab. Invest. 56(5):401-407.
Crossref
|
|
|
|