ABSTRACT
Commercial fermented milks have health benefit potential attributed to pure probiotics used as starter culture. Lactic acid bacteria (LABs) are the probiotics and promote stabilization of the gastrointestinal microecology of humans by producing secondary metabolites like lactic, acetic and propionic acid, hydrogen peroxide and bacteriocins (bactericidal proteins produced by the lactic acid bacteria). These metabolites are health benefiting. Mursik, an indigenous fermented milk product is consumed at households by many Kenyan communities, for example Kalenjin. The fermentation is not based on pure cultures, instead it is spontaneous. Whether the type and concentration of the probiotics in this traditionally fermented milk have the same probiotic potential as those of commercial fermented milk is not documented. This study aimed at isolating mursik probiotics and determining their probiotic potential. Mursik was obtained from informal women groups and individuals involved in small scale production and marketing in Bomet County, Kenya. Forty one (41) samples of mursik were collected. Probiotic isolation was done by pour plate method and the probiotic potential was done by using disc diffusion method against Salmonella enteritica ATCC 13076, Escherichia coli ATCC 25922 and Staphylococcus aureus isolate which were used as standard strains of public health concern. Data was analyzed using analysis of variance (ANOVA) and chi-square tests at α = 0.05. The main probiotics isolated from mursik include Lactobacillus plantarum, Lactobacillus fermentum, Lactobacillus brevis and Lactobacillus casei. The metabolites produced by the isolated probiotics in broth demonstrated significant (P<0.05) antibacterial effect against the standard strains used. The results obtained from this study confirm that the probiotics in mursik, a traditionally fermented milk product have significant potential against enteric and environmental pathogens that are of public health concern.
Key words: Mursik, probiotic, lactic acid bacteria, antibacterial activity, Lactobacillus.
The part of fermented milk in human being diet is well accredited and the plusses of these products were known to man even during the ancient days of civilization. These products have long been an important component
of nutritional diet. The medicinal and nutritional properties of various fermented foods have been experienced by several generations. However, the scientific community gave impetus to these beliefs and suggested consumption of lactobacilli fermented milk to prolong life (Hansen and Sartor, 2015; Ashton, 2013). Further desirable bacteria in fermented milk could help in suppressing the undesirable and disease causing bacteria in the intestine of human beings (Huttenhower et al., 2014; Ashton, 2013). In Kenya, traditional fermented milk products are mainly produced by pastoral communities such as the Maasai, Borana, Kalenjins and Somali. They are mainly produced by spontaneous fermentation of the milk in traditional containers such as gourds and skin bags.
Some of these fermented milks have been reported to have health beneficial properties such as Kule naoto produced by the Maasai, Mursik produced by the Kalenjin (Muigei et al., 2013; De Vuyst and Leroy, 2007) and Suusac produced by the Borana and Somali (De Vuyst and Leroy, 2007). Mursik is mainly produced by the Kalenjin community in Kenya, through spontaneous fermentation of cow milk in a traditionally prepared gourd (Muigei et al., 2013), referred to as ‘Sotet’ in Kalenjin. It forms a major part of the Kalenjin diet due to its delicious taste and belief that it improves health. The Kalenjin community also values it as a special drink that is shared in special occasions to symbolize success of certain activities such as successful marriage negotiations and weddings, victory in athletics among other events (Muigei et al., 2013). Fermented milk product has been defined by the International Dairy Federation as the milk product prepared from skimmed milk or not with specific cultures (Elaine and Danilo, 2012).
The microflora is kept alive until sale or consumption by the consumers and may not contain any pathogenic germs. Lactic acid bacteria (LAB) widely dispersed in the nature and occurring native microflora in fermented milk that plays an important role in improving their increased shelf life. Within the LAB group, the genus Lactobacillus is the most widely encountered for probiotics because they display numerous antimicrobial activities. This is mainly due to the production of antimicrobial metabolites including organic acids, hydrogen peroxide, and bacteriocins. Among these, bacteriocins have gained increasing interest. Bacteriocins, as defined by Coelho et al. (2014) and Hammes and Vogel (1995) are composites produced by bacteria that unveil a bactericidal mode of action against related as well as unrelated organisms. Bacteriocin generally exert their antimicrobial action by interfering with the cell wall or the membrane of target organisms, either by inhibiting cell wall biosynthesis or causing pore formation, subsequently resulting in death (Bastos et al., 2015; Ogunbanwo et al.,2003; Sullivan et al., 2002).
The highly promising results of these studies underline the important role that functional LABs strains may play in the food industry to improve food quality and safety (De Vuyst and Leroy, 2007). Commercial yoghurt and fermented milk products are the main source probiotics. They contain Lactic acid bacteria of known concentration and type. The functional properties and safety of these lactic acid bacteria have been extensively studied and their efficiency for health benefits. However, probiotics from spontaneous traditional fermented milk like mursik has not been characterized and that their potential as health benefiting is unclear. Therefore, the study isolated Lactobacillus strains as probiotics from mursik of Kenya in prevention of enteric bacterial infections.
Study area
The samples for the study were obtained from Bomet County since it comprises of high production and consumption of fermented and non-fermented cow milk. The county has a prominent peri-urban cow population and thriving cow milk business (Muinde, 2011). The county covers approximately 1,592.4 km2 and lies between latitudes -0.78333 (latitude in decimal degrees) south of the equator and between the longitudes 35.35 (Longitude in decimal degrees) east of Greenwich Meridian. Dairy farming is carried out in the county on both small scale and large scale that supply milk and milk products to Bomet town and other towns in the county (Muinde, 2011). This provides residents with a source of income to supplement other sources such as horticulture and tea farming.
Samples collection and technique
A total of forty one samples of traditionally fermented, mursik were collected from informal women groups in Bomet, Kenya. Snowballing technique was used to identify the women groups involved in mursik production and consent obtained from the group leaders on sample collection. The samples were then transported in ice box to Dairy, Food Science and Technology Department, Egerton University, Food Microbiology Laboratory for analysis.
Statistical analysis
The experiment was carried out in a completely randomized design (CRD). Mursik samples were collected randomly from women groups. Data obtained was computed to analysis of variance (ANOVA) using SPSS version 20 and least significant difference (LSD) test used to test significant differences at α = 0.05.
Isolation of Lactobacillus species
Pour plate technique was also used to isolate the organisms. Samples were serially diluted; 10 g from each mursik sample was transferred aseptically into 90 g sterile 2% (w/v) peptone water (CDH) solution and mixed thoroughly. Serial dilutions of up to10-6 to 10-7 were subsequently made using sterile peptone water. 1 ml aliquot of the samples and dilutions were plated into de-Mann, Rogosa and Sharpe (MRS) agar (HIMEDIA) (pH 6.2 and 5.5). The plates were incubated at 30°C for 48 h under anaerobic conditions (in anaerobe jar using Oxoid anaerogen compact). The use of these medium aimed to isolate and enumerate lactobacilli. Colonies with distinct colonies such as colour, shape and size were selected and purified by streaking three times in MRS Agar (Ashton, 2013).
Identification of Lactobacillus spp.
All strains were maintained by weekly sub culturing on MRS Agar. All tests were carried out from 48 h MRS Agar cultures. The morphological characteristics of isolates were examined after staining by Gram stain. Growth characteristics were monitored daily at 10, 15 and 45°C in tubes of MRS broth (HIMEDIA) over 7-day period. Salt tolerance was assessed after 3 days of incubation at concentration of 4, 6.5, 8 and 10% NaCl in MRS broth. Catalase test was carried out by transferring a drop of MRS broth culture onto a clean slide. To the culture, a drop of H2O2 was added and observed for production of effervescence (Barakat et al., 2011). CO2 production from glucose using citrate lacking MRS broth and inverted Durham tubes were observed at 35°C for 5 days. Production of ammonia from arginine was detected using Nessler’s reagent.
Carbohydrate fermentation
All strains were grown overnight at 37°C in MRS broth, but glucose and meat extract were omitted. Solution of 1% (w/v) of the test carbohydrate were sterilized by membrane filtration (0.2 µm cellulose filtrate, Europe PN: 514-0061) and added to sterilized medium at final concentration of 20 g/L. Carbohydrate utilization was assessed at 24, 48 and 168 h after incubation of the test isolate at 37°C (Bezkorvainy, 2001).
Determination of antimicrobial activity
The antagonistic properties of isolated LABs species were determined by modifying the disc diffusion method. Sterile blotting paper discs (10millmetres) were dipped into 48 h incubated Lactobacillus spp. culture broth and then placed on solidified Nutrient Agar seeded with 3 h old culture of test pathogens, which included Salmonella enteritica (ATCC 13076), Escherichia coli (ATCC 25922) and Staphylococcus aureus (Lab. Isolate). The plates were kept at 4°C for 1 h diffusion and then incubated at 37°C for 24 h. Zones of inhibition were measured (Bauer et al., 1966).
Acid and bile salt tolerance
Isolated Lactobacillus spp. were inoculated into MRS medium of varying pH, that is, 2, 3, 4 and 5 as well as broth with varying concentrations of bile salt (oxgall, 0.5, 1.0, 1.5, and 2.0% w/v), and incubated at 37°C for 48 h. Then 0.1 ml inoculums was transferred to MRS agar by pour plate method and incubated at 37°C for 48 h. The growth of LABs on MRS agar plate was used to designate isolates as acid or bile salt tolerant (Klaenhammer and Kullen, 1999).
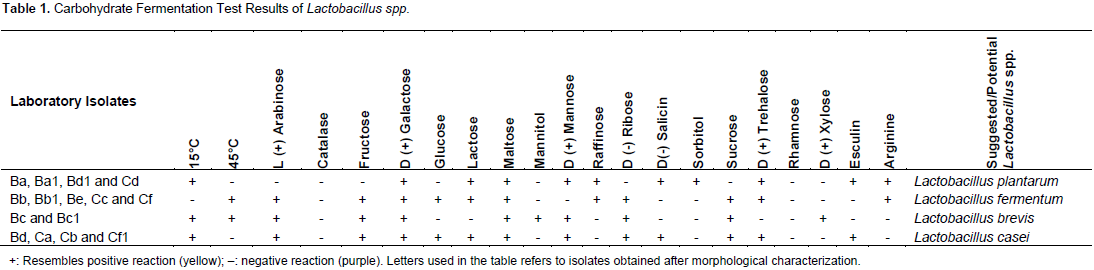
A total 41 mursik samples were analyzed from Bomet and from the samples 64 isolates were obtained. However, 16 isolates remained at the end of the isolation, purification after the loss of unstable isolates during purification and sub culturing steps. All of the isolates were gram positive and catalase negative rods. It was understood that the isolates from fermented milk were so sensitive to the sub culturing. According to the carbohydrate fermentation test results of the 16 isolates Ba, Ba1, Bd1 Cd and Ce gave positive results with the carbohydrates, galactose, lactose, mannose, raffinose, salicin, sorbitol, trehalose, and esculin and gave negative results with xylose, ribose, arabinose, mannitol, raffinose, maltose, sucrose, fructose, lactose, glucose and rhamnose. Bb, Bb1, Be, Cc and Cf gave positive results with ribose, arabinose, trehalose, raffinose, galactose, maltose, sucrose, lactose, glucose and fructose. Isolates Bc and Bc1 gave positive results with xylose, ribose, arabinose, mannitol, galactose, maltose, sucrose, mannose and fructose.
Isolates Bd, Ca, Cb and Cf1 gave positive results on ribose, arabinose, trehalose, galactose, maltose, mannose, fructose, lactose, glucose, esculin and salicin. The isolates gave different fermentation patterns when they are compared. The patterns are shown in Table 1. When the carbohydrate utilization test results were compared with the literature information, it seems that isolates Ba, Ba1, Bd1 and Cd were similar as Lactobacillus plantarum; Bb, Bb1, Be, Be1, Cc and Cf, as Lactobacillus fermentum; Bc and Bc1 as Lactobacillus brevis; and Bd, Ca, Cb and Cf1 as Lactobacillus casei (Roos et al., 2005; Hammes and Vogel, 1995; Bergey et al., 1989). Some similar Lactobacillus spp. were isolated from traditional dairy products of Ardabil region in Iran by Jafari et al. (2011). Antimicrobial effects of LAB were attributed to some substances such as the undissociated acids and production of other primary and secondary antimicrobial metabolites.
To date, different studies show that different species produce different antimicrobial substances which are affected by factors of the species of microorganisms, ingredients and pH of medium, incubation temperature and time (Mohnl et al., 2007). All the Lactobacillus strains isolated were examined according to their antimicrobial activity. In this test, all the test strains were inhibited indicating that the inhibitory metabolites produced by the isolates were extracellular and diffusible because inhibition took place by diffusing on the agar layer. All the four suggested that potential isolates inhibited the growth of the test strains to varying degrees (Table 2 and Figure 1). The four Lactobacillus spp., L. fermentum, L. plantarum, L. brevis and L. casei, inhibited the growth of the test strains to varying degrees. Salmonella enteritica was the most sensitive to Lactobacillus isolates with mean diameter of 17.1 ± 1.1 mm. The highest diameter of 17.4 ± 0.4 mm was observed on Lactobacillus casei while the lowest diameter of 15.3 ± 0.1 mm for L. brevis against S. enteritica. E. coli was moderately inhibited in the study with the highest diameter of 17.1 ± 0.1 mm was obtained from Lactobacillus brevis followed by L. plantarum, L. casei and L. fermentum with zones of inhibition at 16.9, 16.6 and 16.5 mm, respectively.
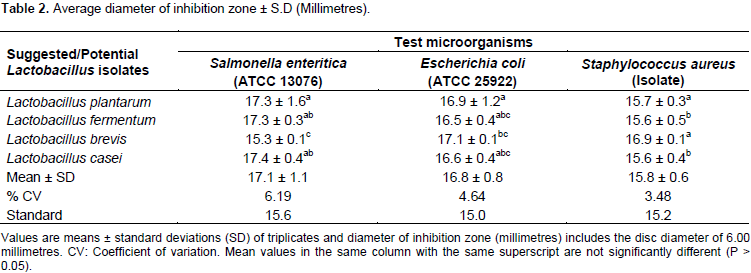
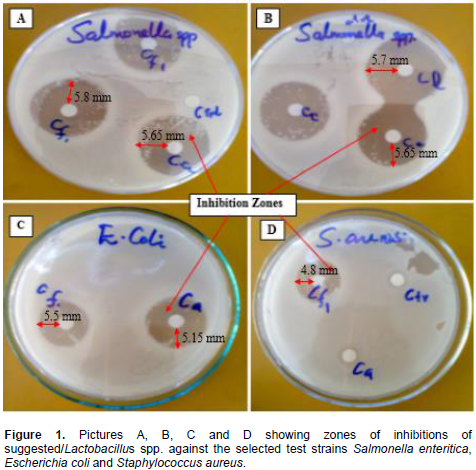
Tests against S. aureus isolate was observed to exhibit small zones of inhibitions when compared with the other test strains, the zones of inhibition against ranged from 15.6 to 16.9 mm with L. casei showing the least zone of inhibition while L. brevis showing the highest zone of inhibition against Staphylococcus aureus. These varying degree of inhibition could be due to metabolic pathways controlled by the test microorganisms gene expressions such as acid-tolerance response system that protects them against severe acid stress for longer periods, hence showing that E. coli (ATCC 25922) and S. aureus being more tolerant to some organic acids than S. enteritica (Dardir, 2012). When mean of the inhibition zones produced by isolates in specific test microorganisms were compared, differences were significant (p < 0.05). Likewise, significant difference was observed in degree of inhibition between the control and the LAB strains in all cases (p < 0.05). Based on the differences in inhibition zones produced by LABcultures and the control, the highest inhibition was observed in the test strains S. enteritica followed by E. coli and S. aureus in that order.
Among the three test strains used in this study, S. enteritica subsp. enteritica serovar enteritidis (ATCC 13076) was relatively susceptible to the antimicrobial activity of LAB than the Escherichia coli (ATCC 25922) and S. aureus (Isolate). The differences in antagonistic activity of LABs on tested pathogenic organisms may be due to production of antimicrobial compounds such as lactic acid, acetic acid or bacteriocins to a varying degree (Buntin et al., 2008). The antimicrobial effect of lactic acid is due to undissociated form of acid, which penetrates the membrane and liberates hydrogen ion in the neutral cytoplasm thus leading to inhibition of vital cell functions. This might be due to the production of acetic and lactic acids that lower the pH of the medium or competition for nutrients or production of bacteriocins or other antibacterial compounds (Chuayana et al., 2003; Quwehand et al., 2002).
Acid and bile salt tolerance
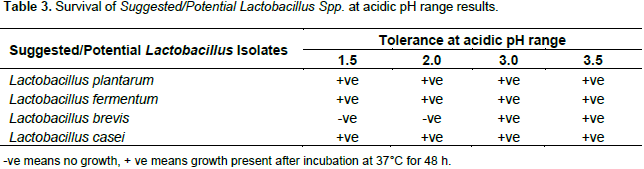
When the isolates were screened whether they could survive at an acidic pH range of between 1.5 and 3.5, the observations made showed that L. plantarum, L. fermentum and L. casei isolates were resistant to low pH except for the L. brevis isolate which was not resistant to pH at 1.5 and 2.0. However, all the isolated Lactobacillus strains were tolerant to pH 3.0 and 3.5 (Table 3). Growth in acidic conditions by the isolates can be considered as significant character of probiotics. Because in intestinal tract, acidic pH is there and isolates should survive from the entry and to establish in gut. Survival of fermented milk bacteria in the presence of acid is significant because in case of human consumption of fermented milk, they might colonize stomach with friendly bacteria (Srinu et al., 2012; Maragkoudakis et al., 2005). This suggests that bile resistant traits of Lactobacillus spp. are crucial for maintaining viability during gastrointestinal transit and are desirable attributes of an orally administered probiotic. Moreover, it should be mentioned that probiotic lactic acid bacteria are mostly consumed in fermented dairy products and milk proteins may provide a protective martrix enhancing and supporting survival of bacteria in the gastric juice of the stomach (Ouwenhand et al., 2001; Fernandez et al., 2003; Tambekar and Bhutada, 2010).
Just like resistance to acidic pH tolerance to bile was considered as a prerequisite for colonization and metabolic activity of bacteria in intestine of the host (Dardir, 2012; Zoumpopoulou et al., 2008). In the study, it was observed that all the LAB strains survived and tolerated bile salts (Oxgall) concentrations of 0.3 to 2.0% quite effectively. But a marginal decrease in viability of all the strains was found when the bile salt concentration was increased from 0.3 to 2.0% (Figure 2). The differences in the absorbance values of bile tolerance (at 620 nm) between strains in this study might be due to differences in their ability to grow and colonize, as the bile salt concentration was increased from 0.3 to 2.0%. The ability of strains to de-conjugate bile acids was decreasing, hence the marginal decreased. Similar to this study, marginal decrease in viability has been reported by Barakat (2011) and Shukla et al. (2010).
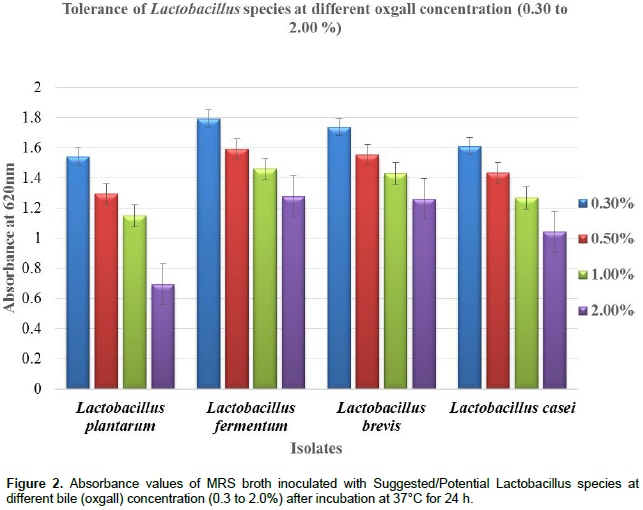
Among all the suggested/potential Lactobacillus isolates, isolate L. fermentum showed the highest absorbance at 620 nm at different bile salt concentrations ranging between 0.3 and 2.0% (w/v). Implying that L. fermentum is assumed in the study to be the most effective strain in de-conjugation by producing high amounts of bile salt hydrolase (BSH) activity on bile acid reducing their toxic effects when compared with other isolates. Bile salt hydrolase activity or possession of the bile salt hydrolase homologs in their genetic material has most often been found in organisms isolated from the commensal inhabitants of the gastrointestinal tract such Lactobacillus, Bifidobacterium, Enterococcus, and Bacteroides species and not in the bacteria isolated from environments from which bile salts are absent (Holzapfel, 2002; Tanaka et al., 1999; Rana et al., 2011). Property of tolerance to bile concentrations and acidic pH could be advantageous for probiotic culture for successful colonization in gastrointestinal environment.
This study indicated that the suggested/potential isolated Lactobacillus spp. meet several of the criteria for use as a probiotic. These characteristics may be advantageous for a probiotic culture to be successful in colonizing and competing with pathogens in gastrointestinal environment. The isolated probiotics have the ability to survive on acidic conditions, tolerate bile, and produce antimicrobial compounds that are active against enteric pathogens of public health concern. Probiotic approach is to reconstitute natural condition by means of repairing a deficiency either by producing organic acids, antimicrobial substance, vitamins, etc., and remove foreign chemicals from the body, which may have toxic consequences or as in the case of antibiotics induce resistance and compromise subsequent therapy.
The authors have not declared any conflict of interests.
The authors wish to thank all women of Bomet County, Kenya for their assistances during collection of mursik samples.
REFERENCES
|
Ashton QA (2013). Lactobacillus – Adv. in Res. and App. Atlanta, Georgia: Scholarly Editions.
|
|
|
|
Barakat OS, Ibrahim GA, Tawfik NF, El-kholy WI, Gad el-rab DA (2011). Identification and probiotic characteristics of Lactobacillus Strains isolated from traditional domiati cheese. Int. J. Microbiol. Res. 3:59-66.
Crossref
|
|
|
|
|
Bastos MCF, Coelho MLV, Santos OCS (2015). Resistance to bacteriocins produced by Gram-positive bacteria. Microbiology 161:683-700.
Crossref
|
|
|
|
|
Bauer AW, Kirby WM, Sherris JC (1966). Antibiotic susceptibility testing by a single disc method. Am. J. Pathol. 45:493-496.
|
|
|
|
|
Bergey DH, Harrison FC, Breed RS, Hammer BW, Huntoon FM (1989). Bergey's Manual of Determinative Bacteriology (Vol. 9). London: William and Wilkins Company.
|
|
|
|
|
Bezkorvainy A (2001). Probiotics: Determinants of survival and growth in the gut. Am. J. Clin. Nutr. 73:399-405.
|
|
|
|
|
Buntin N, Chanthachum S, Hongpattarakere T (2008). Screening of lactic acid bacteria from gastrointestinaltracts of marine fish for their potential use as pro-biotics. Songklanakarin J. Sci. Technol. 30:141-148.
|
|
|
|
|
Chuayana EL, Ponce C, Rivera RB, Cabrera E (2003). Antimicrobial activity of probiotics from milk products. Phil. J. Microbiol. Infect. Dis. 32(2):71-74.
|
|
|
|
|
Coelho MLV, Coutinho BG, Santos OCS, Nes IF, Bastos MCF (2014). Immunity to the Staphylococcus aureus leaderless four-peptide bacteriocin aureocin A70 is conferred by AurI, an integral membrane protein. Res. Microbiol. 165:50-59.
Crossref
|
|
|
|
|
Dardir HA (2012). In vitro evaluation of probiotic activities of lactic acid bacteria strains isolated from novel probiotic dairy products. Glob. Vet. 8(2):190-196.
|
|
|
|
|
De Vuyst L, Leroy F (2007). Bacteriocins from lactic acid bacteria: production, purification and food application. J. Mol. Microbiol. Biotechnol. 13(4):1-6.
Crossref
|
|
|
|
|
Elaine M, Danilo M (2012). Diversification booklet number 21: Traditional fermented food and beverage for improved livelihoods. Rome: Food and Agriculture Organization (FAO) of the UN.
|
|
|
|
|
Fernandez MF, Boris S, Barbes C (2003). Probiotic properties of human lactobacilli strains to be used in the gastrointestinal tract. J. Appl. Microbiol. 94:449-455.
Crossref
|
|
|
|
|
Hammes WP, Vogel RF (1995). The genus Lactobacillus. The Genera of Lactic Acid Bacteria. UK.: B.J.B. Wood Blackie Academic and Professional.
|
|
|
|
|
Hansen JJ, Sartor RB (2015). Therapeutic manipulation of the microbiome in IBD: current results and future approaches. Curr. Treat. Options Gastroenterol. 13(1):105-20.
Crossref
|
|
|
|
|
Holzapfel WH (2002). Apt starter culture technologies for small-scale fermentation in developing countries. Int. J. Food Microbiol. 75(3):197-212.
Crossref
|
|
|
|
|
Huttenhower C, Kostic AD, Xavier RJ (2014). Inflammatory bowel disease as a model for translating the microbiome. Immuniy 40:843-854.
Crossref
|
|
|
|
|
Jafari B, Rezaie A, Siamak A (2011). Isolation and Identification of potentially probiotic bacteria from Traditional Dairy Products of Ardabil region in Iran. Ann. Biol. Res. 2(6):311-317.
|
|
|
|
|
Klaenhammer TR, Kullen MJ (1999). Selection and design probiotics. Int. J. Food Microbiol. 50(1):45-57.
Crossref
|
|
|
|
|
Maragkoudakis PA, Zoumpopoulou G, Miaris C, Kalantzopoulos G, Pot B, Tsakalidou E (2005). Probiotic potential of Lactobacillus strains isolated from dairy products. Int. Dairy J. 16:189-199.
Crossref
|
|
|
|
|
Mohnl M, Acosta AY, Ojeda AA, Rodriguez SB, Pasteiner S (2007). Effect of synbiotic feed additive in comparison to antibiotic growth promoter on performance and health status of broilers. Poult. Sci. 86(1):217.
|
|
|
|
|
Muigei SC, Shitandi A, Muliro P, Ogata RB (2013). Production of Exopolysaccharides in the Kenyan Fermented Milk, Mursik. Int. J. Sci. Res. ISSN (Online):2319-7064.
|
|
|
|
|
Muinde A (2011). Popular milk, mursik now in shops. Retrieved June 14, 2016, from Farmbiz Africa:
View
|
|
|
|
|
Ogunbanwo S, Sanni A, Onilude A (2003). Influence of cultural conditions on the production of bacteriocins by Lactobacillus brevis OG1. Afr. J. Biotechnol. 2(7):179-184.
Crossref
|
|
|
|
|
Ouwenhand AC, Tuomola EM, Tolkko S, Salminen S (2001). Assessment of adhesion properties of novel probiotic strains to human intestinal mucus. Int. J. Food Microbiol. 64:119-126.
Crossref
|
|
|
|
|
Quwehand A, Salminen S, Isolauri E (2002). Probiotics: an overview of beneficial effects. Antonie Van Leeuwenhoek 82(1-4):279-289.
Crossref
|
|
|
|
|
Rana AS, Mehta S, Pandhi ND (2011). Isolation and Characterization of Intestinal Bactria as Potential Probiotics. Functional food - A new concept for sustainable livelihood. Junagardh: Dept. of Biotechnol. Agric. Uni. Junagardh. pp. 117-126.
|
|
|
|
|
Roos S, Engstrand L, Jonsson H (2005). Lactobacillus gastricus sp. nov., Lactobacillus antri sp. nov., Lactobacillus kalixensis sp. nov., and Lactobacillus ultinesiss sp. nov. Isolated from human stomach mucosa. Int. J. Syst. Evol. Microbiol. 55:77-78.
Crossref
|
|
|
|
|
Shukla G, Sharma G, Goyal N (2010). Probiotic Characterization of Lactobacilli and Yeast Strains Isolated from Whey Beverage and Therapeutic Potential of Lactobacillus Yoghurt in Murine Giardiasis. Am. J. Biomed. Sci. 2:248.
Crossref
|
|
|
|
|
Srinu B, Madhava RT, Kondal RK, Venkata MR (2012). Evaluation of probiotic characteristics in certain lactic acid bacterial strains by in vitro techniques. Acta Biol. Ind. 1(2):149-154.
|
|
|
|
|
Sullivan LO, Ross RP, Hill C (2002). Potential of bacteriocin-producing lactic acid bacteria for improvements in food safety and quality. Biochemie 84(5-6):593-604.
Crossref
|
|
|
|
|
Tambekar DH, Bhutada SA (2010). An evaluation of probiotic potential of Lactobacillus Sp. from milk of domestic animals and commercial available probiotic preparations in prevention of enteric bacterial infections. Recent Res. Sci. Technol. 2(10):82-88.
|
|
|
|
|
Tanaka H, Doesburg K, Iwasaki T, Mireau I (1999). Screening of lactic acid bacteria for bile salt hydrolase activity. J. Dairy Sci. 82:2530-2535.
Crossref
|
|
|
|
|
Zoumpopoulou G, Foligne B, Christodoulou K, Grangette C, Pot B, Tsakalidou E (2008). Lactobacillus fermentum ACA-AD 179 displays probiotic potential in vitro and protects against trinitrobenzene sulfonic acid (TNBS)-induced colitis and salmonella infection in murine models. Int. J. Food Microbiol. 121:18-26.
Crossref
|
|