ABSTRACT
A 21-day study was carried out to determine the effect of resin from Commiphora swynnertonii on plasma levels of glucose and lipids together with liver functions in both normal and carbon-tetrachloride-treated chickens. Sixty growing male broiler chickens were randomly assigned into six groups (n = 10). G1 was a negative control, which received distilled water throughout the experiment. G2 was a positive control and received 1.59 g CCl4 per kg body weight orally every other day to induce liver injury. G3 received 500 mg resin per kg body weight daily, while G4, G5 and G6 received 500, 750 and 1000 mg resin per kg body weight respectively daily plus 1.59 g CCl4 per kg body weight every other day, throughout the experiment. Plasma levels of glucose, triglycerides, lipoproteins and total proteins together with liver functions were determined. Results revealed that oral administration of CCl4 caused liver injury with consequent significant increase (P < 0.05) in the plasma glucose and lipid levels. Also a significant increase (P < 0.05) in the activities of liver enzymes (P< 0.01) and a decrease in total plasma protein was evident. On the other hand, oral administration of moderate dose of the resin reversed these effects by significantly lowering plasma glucose and lipid levels while decreasing liver enzyme activities and boosting plasma protein levels. It is concluded that administration of moderate levels of C. swynnertonii to chickens was capable of reducing plasma levels of glucose (hypoglycemic effect) and lipids (hypolipidemic effect), while protecting the liver against the injury caused by CCl4 (hepatoprotective effect). These findings could have a positive implication on the use of the C. swynnertonii resin for treating diabetes and cardiovascular diseases in humans.
Key words: Commiphora swynnertonii, carbon tetrachloride, liver injury, hepatoprotective effect, plasma glucose, plasma lipids, chickens.
Over centuries, plants have been broadly used as an important source of medicines for both human and (Ruffo et al., 2002; Saimo et al., 2003). Traditional herbal formulations are regarded as an alternative to modern Traditional herbal formulations are regarded as an alternative to modern medicine and are used globally as the primary means of health care (Kayombo et al., 2013). In developing countries, more than 70% of the population depends on traditional medicine for primary healthcare (Kayombo et al., 2013). Similar global estimates are reported by World Health Organization (WHO, 1996), implying that the primary healer to majority of the global community is a plant. Various plants in the genus Commiphora have been studied and found to have several medicinal utilities including antidiabetic, antioxidant, anti-inflammatory and antimicrobial activities, among others (Shen et al., 2012; Hassan et al., 2013; Bakari et al., 2015). The efficacy of resins obtained from Commiphora mukul and Commiphora molmol against hyperlipidemia and schistosmiasis have been substantiated by pharmacological results and therefore validated for the treatment of the diseases in Egypt and India (Shen et al., 2012). In addition, guggulsterone, a bioactive steroid compound extracted from resin of C. mukul, demonstrated both hypoglycemic and hypolipidemic effects against high fat diet-induced diabetes mellitus in rats, with plausible mechanisms (Yang et al., 2012), suggesting its usefulness in treating type 2 DM (Sharma et al., 2009). Some studies have reported guggulsterone to have a potent inhibitory activity against tumor cells and inflammation (Shen et al., 2012). Commiphora swynnertonii is less reported although it is used by Maasai and other local community people in Tanzania and Kenya (Kalala et al., 2014). The current study therefore, aimed at investigating the effect of resin from C. swynnertonii on plasma glucose and lipids levels and liver functions using chicken model.
Commiphora resin and its preparation
The resin was sourced from Simanjiro district in Manyara region (Northeastern Tanzania), located at 4°0′0″ S, 36°30′0″ E and 1,360 m above sea level. Following harvesting of the resin, subsequent processing was carried out in a laboratory at Sokoine University of Agriculture (SUA) as described by Bakari et al. (2011). 100 g of the resin extract were dissolved into 750 ml of distilled water to make a concentration of 133.33 g/L of the solution and left for 30 min before filtration using a filter paper. Thereafter, the solution was stored in
clean bottles at 4°C until experimentation.
Experimental animals and their maintenance
Twenty one day-old male broiler chickens were purchased from a local seller in Morogoro township and were moved into a brooder house at the university farm where they were dewormed using mebendazole (Kukuzole®, Interchem Pharma Ltd, Moshi Tanzania) and received anticoccidial drug amprolium (Norbrook Laboratories Ltd, UK). The chickens were fed on maize bran-based mash with ad libitum drinking water and after four weeks they were moved into experimental cages for a two week-acclimatization period.
Experimental design
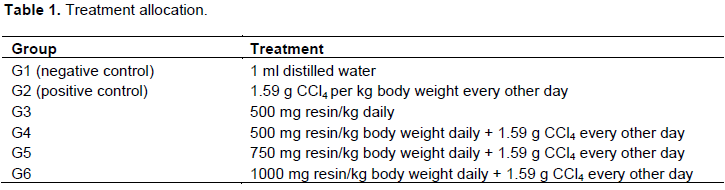
Following acclimatization, the chickens were randomly assigned into six groups (n = 10) and given various treatments orally using 5 ml syringe for 21 days. G1 was a negative control, whereby chickens received distilled water daily throughout the experiment. Chickens in G2 (positive control) were treated with carbon tetrachloride at 1.59 g CCl4 per kg body weight every other day for the purpose of inducing liver injury, hampering glucose uptake and lipid metabolism. G3 received a daily dose of 500 mg resin per kg body weight. Chickens in G4, G5 and G6 received 500, 750 and 1000 mg resin per kg body weight, respectively daily and 1.59 g CCl4 per kg body weight every other day throughout the experiment (Table 1).
Blood sample collection and preparation
Blood samples were collected from brachial veins using a 5 ml syringe. The samples were then stored in sterile heparin vacutainer tubes. The blood samples were centrifuged at 1300 rpm for 5 min to separate plasma. The plasma samples were stored in a refrigerator (4°C) until they were used for various analyses.
Laboratory analyses
All laboratory analyses were carried out using standard method kits (Erba® Diagnostics, Mannheim, Germany). Plasma glucose and total cholesterol were analyzed according to Trinder (1969) using the Trinder’s end point method. High density lipoprotein-creatinine (HDL-c) was analyzed using phosphotungstic acid, end point method based on the methodology by Burstein et al. (1970). Analysis of triacylglycerols (TAGs) was done by employing colorimetric enzymatic method using glycerophosphate oxidase. The reagent was based on the described by Fossati and Prencipe, (1982) and Mc Gowan et al. (1983). Very low density lipoprotein- creatinine (VLDL-c) and LDL-c were calculated according to Friedewald et al. (1972) from the equations:
[Plasma VLDL-c] mg/dL = [Triacylglycerols] / 5
[Plasma LDL-c] mg/dL = [Total cholesterol] – [HDL-c] – [VLDL-c]
Liver enzyme (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) were determined by the kinetic methods described by International Federation of Clinical Chemistry (IFCC) without pyridoxal phosphate using Erba ® kit. The method is based on the oxidation of NADH to NAD+ during conversion of alanine to pyruvate.
Effect of C. swynnertonii resin and/or CCl4 on plasma glucose
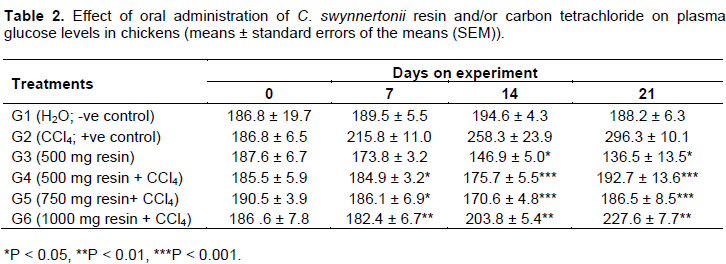
The glucose profile in G1 (negative control group) was not significantly altered throughout the experiment. Chickens in G2 (positive control; CCl4) showed a significant elevation (P < 0.01) in glucose levels with highest levels on day 21 of the experiment. Chickens in G3 (500 mg resin per kg body weight alone) had significantly low plasma glucose (P < 0.01) compared to chickens in G1. Comparison of plasma glucose levels between G2 and each of the groups which received resin and CCl4 (G4, G5 and G6) clearly showed that the resin was significantly effective (P < 0.01) in counteracting the effect of CCl4, that is, by reducing plasma glucose levels (Table 2).
Effect of C. swynnertonii resin and/or carbon tetrachloride treatments on plasma lipids
Triacylglycerols (TAG)
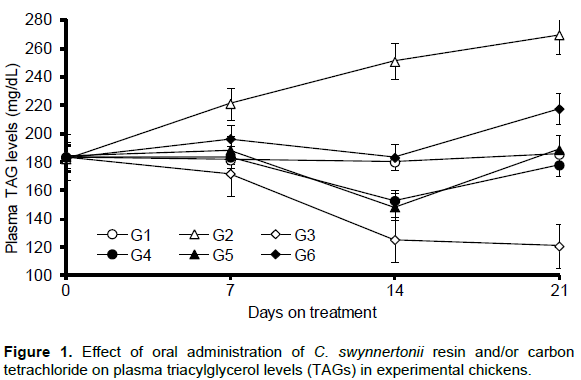
Results of the effect of C. swynnertonii resin and/or CCl4 treatments on plasma TAGs are depicted in Figure 1. There was no significant change (P > 0.05) in the levels of plasma TAGs of the chickens in the negative control group, G1. Oral administration of CCl4 alone to chickens in G2 significantly increased (P < 0.01) plasma levels of TAGs throughout the experiment. On the other hand, administration of C. swynnertonii resin alone to chickens significantly reduced (P < 0.01) the levels of TAGs in the plasma (G3). Plasma levels of TAGs in all groups (G4, G5 and G6), which received both CCl4 and resin were significantly below those of G2 but above G3 (P < 0.01). There was a slight elevation of TAGs in G5 and G6 on day 21 as compared to the data on day 14 in the same groups.
Total cholesterol
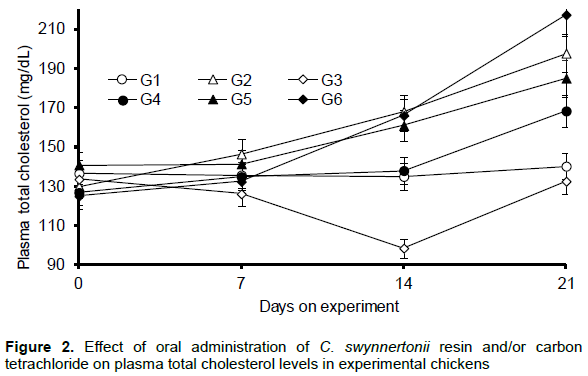
The levels of total cholesterol in the negative control group (G1) did not change significantly throughout the experiment (Figure 2). By day 21, chickens receiving CCl4 alone (G2) had significantly higher (P < 0.05) plasma cholesterol levels in comparison with G1. Chickens receiving resin alone (G3) had significantly lower (P < 0.05) plasma cholesterol as compared to those in G1. G4 had significantly lower (P < 0.05) plasma cholesterol than G2. By day 14 on experiment, there was no significant difference between plasma cholesterol levels of chickens in G5 (750 mg resin) and G6 (1000 mg resin) and that of chickens in G2 (CCl4 alone). However, on day 21 chickens in G6 had the higher plasma cholesterol than those in G2.
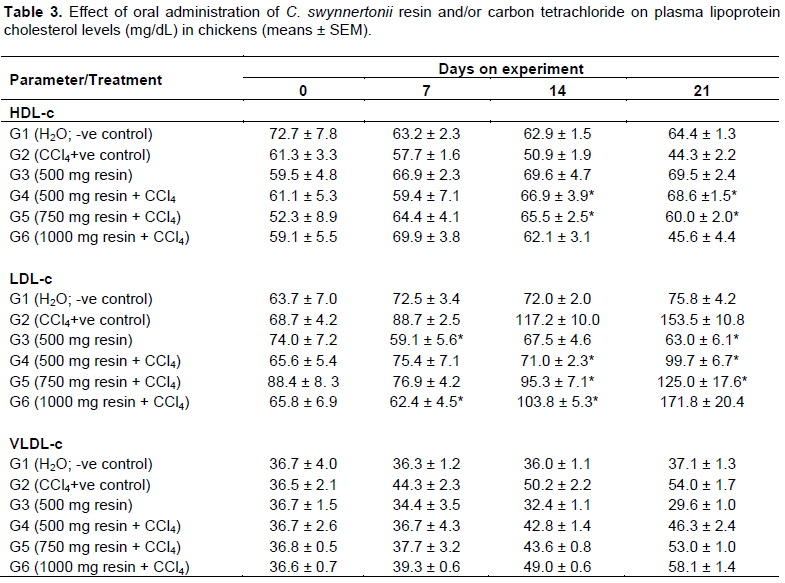
Lipoproteins
Levels of both HDL-c and LDL-c remained unchanged (P > 0.05) in the negative control group G1 while HDL-c significantly (P < 0.01) decreased in the positive control group (G2) as shown in Table 3. Chickens in G3 presented with significant increase in HDL-c compared to other groups (P < 0.05) with comparatively lower LDL-c (P < 0.05). As compared to G2 (positive control), G4, G5 and G6 chickens had significantly higher HDL-c (P < 0.05) by day 14 of experimentation but the levels decreased by day 21, with the decrease in LDL-c levels. The levels of VLDL-c were proportional to those of LDL-c throughout the experiment.
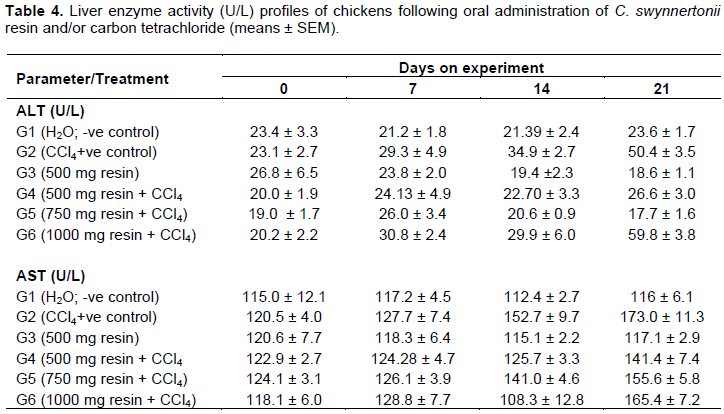
Effect of C. swynnertonii resin and/or carbon tetrachloride treatments on liver enzymes, total bilirubin and protein
Plasma alanine aminotransferase (ALT)
There was no significant difference in the activity of ALT among all the groups on days 0 and 7, but a significant elevation (P < 0.05) in G2 was observed on day 14 (Table 4).
There was a significant fall (P < 0.01) of ALT activity in G3 chickens as compared to G1. Birds in G4 had a significant fall (P < 0.05) in serum ALT on day 14. There was a significant decline (P < 0.01) in the activity of ALT in G5 chickens in comparison to G2. Chickens in G6 had reduced activity of ALT (P < 0.01) on day 14 but the activity increased on day 21 with no significant difference (P > 0.05) from G2.
Plasma aspartate aminotransferase (AST)
The activity of AST did not change significantly in G1
(normal chickens) throughout the experimental period (Table 4). There was significant increase (P < 0.05) in the activity of the enzyme in G2 chickens (CCl4 alone) by day 21. There was no significant difference in the activity of the enzyme between chickens in G1 and those in G3 throughout the observation period. The activity of AST increased steadily in G2 chickens.
There was no significant change of AST activity in G3 chickens throughout the experiment. By day 21, chickens in G4 had significantly lower AST activity than those in G2. Group 5 chickens also showed a significant decrease in AST activity as compared to those in G2. Chickens in G6 had a significant elevation in AST towards the end of the experiment.
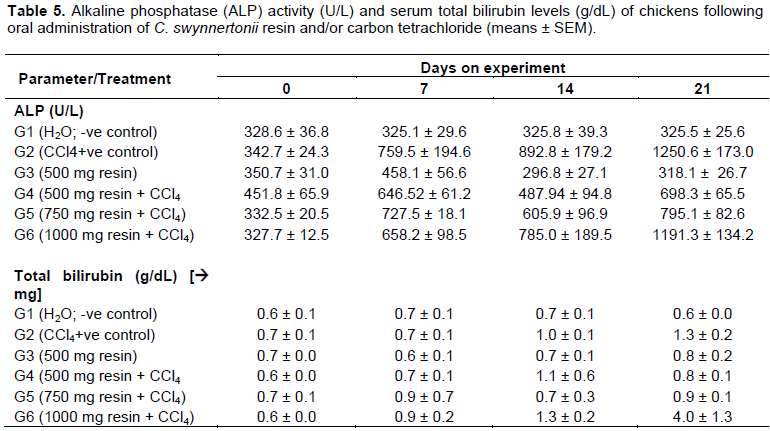
Plasma alkaline phosphatase (ALP) activity and total bilirubin
Serum ALP activity and total bilirubin levels were relatively unchanged in G1 (normal chickens) throughout the experiment (Table 5). Chickens in G2 (CCl4 alone) had highly increased ALP activity (P < 0.01) with a corresponding elevation (P < 0.05) of serum bilirubin by day 21.
Chickens in G3 (resin alone) had no significant change in the activity of ALP compared to those in G1. There was no significant difference in the enzyme activity between chickens in G2 and those in three groups, G4, G5 and G6 by day 14. G4 and G5 chickens showed significantly low ALP activity (P < 0.05) compared to G2 chickens by day 21. G6 chickens had significant increase in bilirubin as compared to those in G2, indicating a three folds-increase.
The increase in bilirubin in G6 was even more than six times that of chickens in G1.
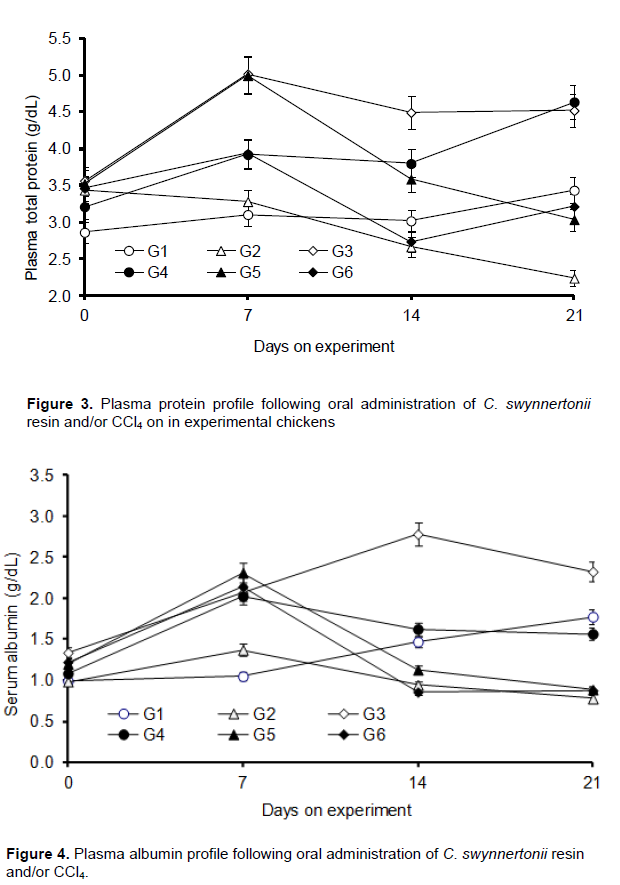
Plasma total protein and albumin
The negative control group (G1) showed no significant change in plasma total protein and albumin whereas the chickens in G2 (CCl4 alone) had significantly lowered (P < 0.05) plasma total protein and albumin (Figures 3 and 4). There was a significant increase (P < 0.01) in plasma protein and albumin in G3 chickens (resin alone) as compared with those in the negative control. Chickens in G4 exhibited a significant increase in plasma total protein (P < 0.01) with a corresponding increase in plasma albumin (P < 0.05) compared to G2 chickens. G5 also had increased plasma total protein (P < 0.05) but with no significant increase (P > 0.05) in albumin levels compared to chickens in G2. Chickens in G6 showed no significant difference (P > 0.05) in the plasma levels of both parameters.
Effect of C. swynnertonii resin on plasma glucose levels
Results of the current study clearly showed that oral administration of moderate doses of C. swynnertonii resin (500 to 750 mg/kg body weight) was able to significantly lower plasma glucose levels in both normal and CCl4 treated chickens. The hypoglycemic effect of Commiphora species has also been demonstrated in other studies using rats (Al-Amoudi, 2010) and chickens (Bakari et al., 2013). In the rat study, it was found that Commiphora myrrh resin was capable of lowering serum glucose levels by 37% in alloxan-induced diabetic rats. In other rat studies (Goji et al., 2009), ethanolic extracts from stem barks of Commiphora africana were shown to induce hypoglycemia in normal rats. Ramesh and Saralakumari (2012) demonstrated significant reduction in plasma glucose in Wistar rats with fructose-induced type 2 diabetes mellitus following oral administration of C. mukul gum resin. The effect of Commiphora spp. resin on plasma glucose has been attributed to various phytochemicals found in the extract such as, alkaloids, terpenoids as well as phenolic compounds (Rauter, 2010; Brahmachari, 2011; Mahmoud 2013, Arif et al., 2014). The possible mechanisms underlying the hypoglycemic activity of resin from C. swynnertonii are still elusive. Some studies using other Commiphora spp. relate the hypoglycemic effect of the Commiphora spp. extracts to mechanisms that decrease precursors of glucose in the liver and increased insulin production by activating pancreatic β-cells (Goji et al., 2009; Atta et al., 2014). It is also possible that resin counteracts the effect of hepatocyte membrane peroxidation by CCl4, thereby protecting insulin receptors and thus improving glucose uptake by the hepatic tissue. Moreover, resin from Commiphora spp. may be involved in enhancing glucose uptake and hence catabolism via activation of glucose transporter genes, insulin receptor genes and/or glucose/hexose kinase gene (Yerima et al., 2012).
Effect of resin from C. swynnertonii on plasma lipid levels
The significant reduction in all forms the tested lipids; triglyceride, total cholesterol, low density lipoprotein and very low density lipoprotein cholesterol in this study suggests that C. swynnertonii resin has hypolipidemic effect. Once again the dose range of 500 to 750 mg resin per kg body weight gave the most optimum response. These are in concurrence with those of other studies done on other Commiphora spp. against lipid levels in various test animals. In a study by Adebayo et al. (2006) using C. africana, oral administration of leaf ethanolic extract at a dose of 150 mg per kg body weight in normal rats decreased serum triglyceride levels by about 24%. Al-Amoudi (2010) found that resin from C. myrrh could lower serum total cholesterol, LDL-c and VLDL-c and increase HDL-c in alloxan diabetic rats. In addition, it was found by Wang et al. (2004) that C. mukul can lower total plasma cholesterol as well as LDL-c by preventing the oxidative modification of lipoprotein forms. Furthermore, Bakari et al. (2015), in a similar study, reported a significant decrease in plasma cholesterol when resin from C. swynnertonii was administered to normal chickens.
The presence of ketonic steroidal compounds such as Z- and E-guggulsterones in C mukul resins has been used to account for the hypolipidemic effect observed in many studies (Wang et al., 2004; Siddigui and Mazumder, 2012). Clues on biochemical mechanisms underlying the activity of this extract can be sought by utilizing the knowledge of biochemical pathways involved in cholesterol metabolism. Recent research publications contend that the mechanisms by which resin may reduce cholesterol involve promoting bile acid synthesis from cholesterol by the activity of the enzyme cholesterol 7-alphahydroxylase (CYP7A1) (Urizar et al., 2002; Cui et al., 2003; Deng, 2007). In study by Young et al. (2012), Z-guggulsterone was found to induce choesteryl ester hydrolase (CES1) and bile salt export pump (BSEP) through transactivation. The same study found that Z-guggulsterone was metabolized by cytochrome P450 3A4 CYPA4) and the metabolites increased the induction potency of BSEP, resulting in enhanced bile acid transport and hence decreased cholesterol and VLDL-c.
Effect of C. swynnertonii resin on liver function
Findings in this study have indicated that oral CCl4 had ability to damage liver cells resulting in increased plasma activities of the liver enzymes. The ability of C. swynnertonii resin to decrease the plasma activities of liver enzymes whilst increasing total protein and albumin establishes the role of C. swynnertonii resin in counteracting hepatic tissue damage (reduced activity of liver enzymes) and boosting liver function in CCl4-treated chickens (increased production of albumin and proteins). These findings are similar to those obtained from other studies using Commiphora opobalsamum (Al-Howiriny et al., 2004) and C. mukul extracts (Hassan et al., 2013; Ahmad et al., 2015) in rat model. In both studies, oral administration of different extracts to CCl4-treated albino rats showed a hepatoprotective effect by decreasing the activities of the liver enzymes.
In the present study, it was also observed that the dose of 500 mg resin per kg alone did not affect liver enzyme activities in chickens. However, the 1000 mg dose significantly increased the enzyme activities in CCl4-injured chickens. This interesting observation suggests that lower doses of C. swynnertonii resin have little side effects while higher doses could be detrimental. These findings are in concurrence with the those reported Bakari et al. (2015), who found that moderate doses did not alter the activities of ALT and AST in normal chickens.
The mechanisms involved in the observed hepato-protective effects of the resin in CCl4-treated chickens are not clearly known but it has been speculated from studies using other Commiphora spp. that bioactive ingredients in the resin could act by binding free radicals, hence, preventing their attack to hepatocellular endoplasmic reticulum membrane (Hassan et al., 2013).
Alternatively, the hepatoprotective effect of C. swynnertonii resin may involve activation of the antioxidant pathways via increased activity of antioxidant enzymes such as superoxide dismutase, glutathione reductase and catalase with a net effect of hepatic protection and function boosting (Ojha et al., 2011). The toxic effect of C. swynnertonii at higher doses (for example 1000 mg) could be linked to hemolytic effect of resin (Bakari et al., 2013) which in turn, could increase total bilirubin (Sharma and Sharma, 2001).
From these findings, it is therefore concluded that oral administration of moderate doses of C swynnertonii resin was capable of lowering plasma levels of glucose and lipids, while protecting the liver from harmful effects of carbon tetrachloride. Further studies are required for the validation of the resin in managing glucose and lipids related disorders in animals.
The authors have not declared any conflict of interests.
REFERENCES
|
Adebayo AH, Aliyu R, Gatsing D, Garba IH (2006). Effects of ethanolic leaf extracts from Commiphora africana (Burseraceae) on lipid profile in rats. Int. J. Pharmacol. 2(6):618-622.
Crossref
|
|
|
|
Ahmad A, Raish M, Ganaie MA, Ahmad SR, Mohsin K, Al-Jenoobi F, Al-Mohizea AM, Alkharfy KM (2015). Hepatoprotective effect of Commiphora myrrha against d-GalN/LPS-induced hepatic injury in a rat model through attenuation of pro inflammatory cytokines and related genes. Pharm. Biol. 53(12):1759-1767.
Crossref
|
|
|
|
Al-Amoudi NS (2010). Influence of esparto grass (Cortaderia sellona L.) myrrh (Commiphora myrrha L.), Fenugreek (Trigonella foenum Graecum L.) on reducing serum glucose of alloxan diabetic rats. Int. J. Food Nutr. Public Health 3:171-181.
|
|
|
|
Al-Howiriny TA, Al-Sohaibani MO, Al-Said MS, Al-Yahya MA, El-Tahir KH, Rafatullah S (2004).Hepatoprotective properties of Commiphora opobalsamum ("Balessan"), a traditional medicinal plant of Saudi Arabia. Drugs Exp. Clin. Res. 30(5-6):213-20.
|
|
|
|
Bakari GG, Max RA, Mdegela HR, Phiri EC, Mtambo MM (2013). Efficacy of resinous extract from Commiphora swynnertonii (Burrt) against Newcastle infection in chickens. Int. J. Med Plants Res. 2:156-161.
|
|
|
|
Bakari GG, Max RA, Mdegela, HR, Phiri, EC, Mtambo MM (2011). Antibacterial and antifungal activity of Commiphora swynnertonii (Burt) on Selected Pathogens of Public Health Importance. Res. J. Biomed. Sci. 6:175-179.
|
|
|
|
Bakari GG, Max RA, Mdegela RH, Pereka AE, Phiri ECJ, Mtambo MMA (2015).Effect of resinous extract from Commiphora swynnertonii (Burrt) on t on various physiological parameters in chickens. J. Med. Plants Res. 9(13):462-470.
Crossref
|
|
|
|
Burstein M (1970). HDL cholesterol determination after separation high density lipoprotein. Lipid Res. 11:583.
|
|
|
|
Cui J, Huang L, Zhao A, Lew JL, Jinghua Y, Sahoo S, Meinke PT, Royo I, Pelaez F, Wrught SD (2003). Guggulsterone Is a Farnesoid X Receptor Antagonist in Coactivator Association Assays but Acts to Enhance Transcription of Bile Salt Export Pump. J. Biol. Chem. 278:10214-10220.
Crossref
|
|
|
|
Deng R (2007). Therapeutic Effects of Guggul and Its Constituent Guggulsterone: Cardiovascular Benefits. Cardiovasc. Ther. 25(4):375-390.
Crossref
|
|
|
|
Fossati P, Prencipe L (1982). Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 28:2077-2080.
|
|
|
|
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18:499-502.
|
|
|
|
Goji ADT, Dikko AAU, Bakari AG, Mohammed A, Ezekiel I, Tanko Y (2009). Effect of Aqueous-ethanolic Stem Bark Extract of Commiphora africana on Blood Glucose Levels on Normoglycemic Wistar Rats. Int. J. Anim. Vet. Adv. 1:22-24
|
|
|
|
Hassan NAM, Helala OK, Mansya, AE (2013). Hepatoprotective and Antioxidant Effects of Commiphora against CCl4-Induced Liver Injury in Adult Male Albino Rats. Int. J. Toxicol. Pharmacol. Res. 5:79-86.
|
|
|
|
Kalala W, Magadula J, Mdegela H (2014). Evaluating acaricidal activity of Commiphora swynnertonii (Burrt) bark exudate against common ticts in Tanzania. Int. J. Herbal Med. 2:19-25.
|
|
|
|
Kayombo EJ, Mahunnah RLA, Uiso FC (2013). Prospects and Challenges of Medicinal Plants Conservation and Traditional Medicine in Tanzania.
Crossref
|
|
|
|
Ojha S, Bhatia J, Arora S, Golechha M, Kumari S, Arya DS (2011). Cardioprotective effects of Commiphora mukul against isoprenaline-induced cardiotoxicity: a biochemical and histopathological evaluation. J. Environ. Biol. 32:731-738.
|
|
|
|
Ramesh B, Saralakumari D (2012). Antihyperglycemic, hypolipidemic and antioxidant activities of ethanolic extract of Commiphora mukul gum resin in fructose-fed male Wistar rats. J. Physiol. Biochem. 68:573-582.
Crossref
|
|
|
|
Ruffo CK, Birnie A, Tengnäs B (2002). Edible wild plants of Tanzania.Regional Land Management Unit (RELMA). Technical Handbook Series 27. Nairobi, Kenya. Swedish International Development Agency (SIDA). P 766.
|
|
|
|
Saimo MK, Bizimenyera ES, Bwanika A, Ssebuguzi F, Weny G, Lubega GW (2003). Ethno veterinary practices in Uganda: Use of medicinal plants in treating helminthosis and coccidiosis in rural poultry and goats in Uganda. Bull. Anim. Health Prod. Afr. 51(3):133-138
|
|
|
|
Sharma B, Salunke R, Srivastava S, Majumder C, Roy P (2009). Effects of guggulsterone isolated from Commiphora mukul in high fat diet induced diabetic rats. Food Chem. Toxicol. 47:2631-2639.
Crossref
|
|
|
|
Sharma P, Sharma JD (2001). In vitro hemolysis of human erythrocytes-by plant extracts with antiplasmodial activity. J. Ethnopharmacol. 74(3):239-243.
Crossref
|
|
|
|
Shen T, Li GH, Wang XN, Lou HX (2012). The genus Commiphora: a review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 142:319-330.
Crossref
|
|
|
|
Trinder P (1969). Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Assoc. Clin. Biochem. 6:24-28.
Crossref
|
|
|
|
Urizar NL, Liverman AB, Dodds DT, Silva FV, Ordentlich P, Yan Y, Gozalez FJ, Heyman RA, Mangelsdorf D, Moore DD (2002). A natural product that lowers cholesterol as an antagonist ligand for FXR. Science 296:1703-1706.
Crossref
|
|
|
|
Wang X, Greiberger J, Ldinski G, Kager G, Paigen B, Jugens G (2004).The hypolipidemic natural product Commiphora mukul and its component guggulsterone inhibit oxidative modification of LDL. Atherosclerosis 172:239-246.
Crossref
|
|
|
|
WHO (1996). WHO monographs on selected medicinal plants. pp. 1-279.
|
|
|
|
Yang DJ, Yang D, Shi D, Xiao D, Chen YTC, Black C, Deng R, Yan B (2012). Hypolipidemic agent Z-guggulsterone: metabolism interplays with induction of carboxylesterase and bile salt export pump. J. Lipid Res. 53:529-539.
Crossref
|
|
|
|
Yerima M, Maje IM, Mahdi MA, Haruna ZB, Khan F, Tanko Y (2012). Effects of the stem-bark extract of Commiphora kerstingii Engl. on alloxan-induced hyperglycaemia and fructose induced insulin resistance. Nigerian J. Pharmacol. Sci. 11:80-86.
|