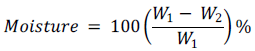The use of enzyme consortium to aid the extraction of shea butter in the traditional village extraction process improves the quality of the shea butter. In this study, shea butter was extracted from three different shea kennel substrates; raw shea kennels (RASK), roasted shea kennels (ROSK) and shea kennel paste (SKEP) from a single variety of shea nuts (Vitellaria paradoxa) using the standard traditional extraction process and aided by 1:1:1 combination of enzymes: lipase, pectinase and cellulase.The characteristics of the resulting shea butter were investigated, and compared to existing recommended standards and export guidelines, using standard methods. The shea butter from raw shea kennels exhibited great qualities with moisture content of 0.18%; the free fatty acid levels of 1.60%; the peroxide valueof3.67 meq/g and the insoluble impurities level of 0.02 %. This generally places enzyme-assisted traditionally extracted shea butter as either Grade 1 or Grade 2. Enzyme-assisted extraction makes the shea butter an export commodity. In summary, shea butter from enzyme aided extraction has improved qualities, is exportable and useful across all the various industries.
Given the occurrence and abundance of shea (Vitellaria paradoxa) trees across the Northern part of Ghana, the nation harbors a huge potential in the shea nut industry. It’s been estimated that 77, 670 square kilometers of land in Ghana are covered by wild growing shea trees (CRIG, 2002). Approximately 60,000 metric tonnes of shea nuts are exported annually representing 30% of the potentially available shea nuts in the northern zones of Ghana (Lovett, 2004). About 70,000 mt of shea nuts processed representing 35% of potentially available nuts are consumed locally (Lovett, 2004). The rest are either left to rot in the bush or are beyond reach. The main focus of the shea industry has been on the butter. Unfortunately, the desired technology that offers the best efficiency is yet to be established. Enzyme-assisted traditional extraction techniques are promising with 70% extraction efficiency achieved in earlier studies (unpublished). The traditional extraction technology is the most widely used technology for shea butter extraction in Africa and particularly in Ghana since several researchers (Alonge and Olaniyan, 2007; Otu et al., 2015; Abdul-Mumeen, 2013; Abdulai, 2015; Larbi and Apea, 2013) have made triumphant attempts to improve upon or find alternatives to the traditional extraction method.
Enzyme-assisted aqueous extractions have been carried out on melon seed, coconut, avocado and shea nuts (Fullbrook, 1983; Buenrostro and Lopez-Munguia, 1986; Tano-Debrah and Ohta, 1995; Otu et al., 2015). Generally enzyme use have been considered environmentally friendly and its use for improving oil extraction efficiency while reducing side products have long been established. Enzymes remain the most efficient way to rupture cell walls even at molecular level (Fullbrook, 1983) but remains particularly convenient for materials with higher oil-to-protein ratio. They can be used together with other methods such as physical or solvent means to extract fats and oils from plant source (Owusu-Ansah, 1997).
Research into enzyme-assisted aqueous extraction of shea butter using different enzymes and targeted at increasing extraction yield has been well documented (Tano-Debrah and Ohta, 1995; Otu et al., 2015). Whether the shea butter from such enzyme-assisted extraction has the desired characteristics that meet international standards is yet to receive the deserved attention. The purpose of this research was in response to this gap.
Shea butter is yellowish-grey solid natural fat extract from the kernel of the shea nut with multifunctional properties. The multifunctional properties of the shea butter depend strictly on its compositional properties: the peroxide value, moisture content, free fatty acid level and the insoluble impurities (UEMOA, 2006).
Shea butter is currently on high demand both locally and internationally (Garba et al., 2015). Locally, the inhabitants of Northern Region of Ghana depend on the shea butter as the valuable natural source of vegetable oil due mainly to its low cholesterol and free fatty acids levels. The butter serves as a source of income for the support of their livelihood. It is also used for cooking, production of local soap and for the treatment of fractures, body pains and other ailments. Internationally, shea butter, depending on its quality, has found extensive application in the pharmaceutical, cosmetic, food and confectionary industries. The purpose however for this research was based on the premise that shea trees, mainly Vitellaria paradoxa, growing wild on the savanna stretch along the northern parts of Ghana are producing nuts that can be enzymatically hydrolysed for shea butter extraction with improved quality. This research sought to examine some physico-chemical properties of enzyme-assisted extracted shea butter.
Samples
Three different types of shea nut samples (substrates) were investigated to showcase the one with the best qualities for use in the various industries. Shea butter was extracted from dried pre-treated shea nut kernels called raw shea kernels (RASK) basically to determine whether roasting before extraction, as it’s always done, was still necessary determinant of shea butter quality. The other two substrates used were roasted shea kernels (ROSK) and roasted shea kernel paste (SKEP) as shown (Figure 1).
Raw shea kernels
The raw shea kernels represent the final product for the initial pretreatment of the shea nut fruit for butter production. It can be stored and used anytime up to two years from its production date. When ripe shea nut fruits fell from gravitational impact, they were picked and depulped. The nuts were boiled and dried before removal of the shells. The raw shea kernels (Figure 1a) were produced.
Roasted shea kernels
The roasted shea kernels (Figure 1b) were produced when the raw shea kernels (Figure 1a) underwent pulverization using the mortar and the pestle or sometimes stone crushers. The pulverized shea kernels were further heated on a dry hot pan/metallic oven to achieve lowest moisture levels and stored as shown in Figure 1b for the shea butter extraction.
Shea kernel paste
The obvious difference between the roasted kernels and the shea kernel paste was the particle size. The shea kernel paste (Figure 1c) was obtained from milling the roasted shea kernels (Figure 1b) to fine particles.
Substrate moisture content analysis
The moisture content (%) of the substrates was determined according to Mohagir (2014) recommended by AFNOR (1981). Five grams (5 g) of ground raw kernels was placed in a dry oven dish, and then dried in an oven at 105±2°C until a constant mass was achieved. The experiment was repeated three times and the average value was taken. The moisture content (Mc) was calculated on a dry matter basis and expressed in percentage of initial sample using the formula below:
Where, m1 is the mass of the sun dried kernels and m2 is the mass of the oven dried kernels.
Commercial enzymes
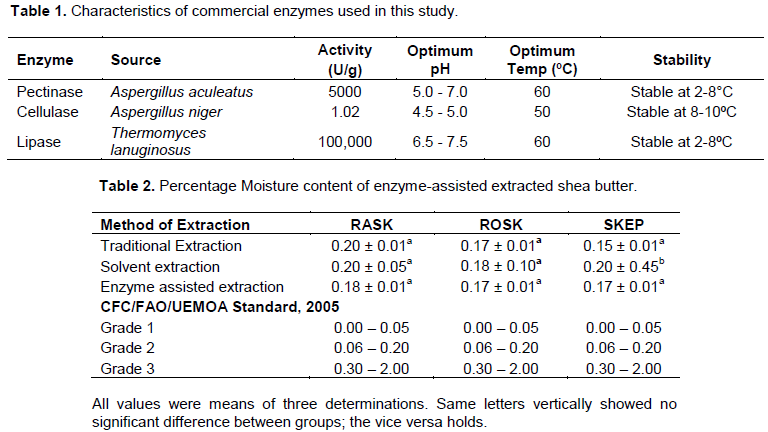
Commercial pectinase (E6287) from Aspergillus aculeatus, Commercial Lipase (E0777) from Thermomyces lanuginosus obtained from Sigma-Aldrich and Commercial Cellulase from Aspergillus niger were used in the analysis. The table below shows the characteristics of the enzymes used in the study.
Prior extraction of enzyme-assisted extracted shea butter
Prior to the characterization of the enzyme-assisted extracted shea butter, the butter was pre-extracted by the traditional extraction method from three different substrates in a laboratory setting. The enzyme-assisted traditional extraction was carried out according to Otu et al. (2015) but not without modifications. An aliquot equivalent to 50 g of Shea nut biomass in 600 ml beaker was stirred with water in the ratio of 1:4 wt/vol. The content of the flasks were gently boiled for 5 min, cooled to about 30°C and then extracted at 0.5, 1.0, 2.0, 3.0 and 4.0% enzyme-substrate concentration. The optimization of enzyme-assisted traditional extraction of butter from shea nut biomass was carried out for enzyme concentration, incubation temperature and incubation period. Aluminum foil (Everpack products, Ghana) was used to cover the flasks and then placed in a water bath shaker (Thermo Fisher Scientific manufacturer) and incubated at different temperatures (40, 50, 60 and 70°C) and time (1, 2, 3 and 4) h, shaking at 80 rpm. Different set of samples were prepared as control (without enzymes) and incubated along-side the test samples for the same period of time. The reaction was terminated at 100ºC and the digests were transferred into 600 ml beakers and extracted using the traditional extraction procedure according to Tanoh-Debrah and Ohta (1995).
Hundred milliliters (100 ml) of hot water was added to the mixture and vigorously stirred before cold water was added to reduce the temperature of the mixture to about 30 to 40°C. The mixture was left to stand overnight to settle. The emulsion which formed the top layer was collected into another beaker. Fresh warm water was added and stirred to wash the emulsion and allowed to settle again. The clean emulsion was collected into a beaker and gently boiled until clear oil was obtained. The oil was decanted into another beaker and placed in an oven at 100°C for about 1 h to dry and clarify the butter. It was then decanted into a weighed aluminum dish, cooled and weighed to estimate the percentage yield.
Solvent extraction as a control method
Solvent extraction of shea butter was carried out to serve the purpose of a positive control and done according to the procedure adopted by the AOAC (1984). The RASK, ROSK and the SKEP were subjected to fat extraction by placing (2 g) each of the samples into cellulose-paper cone and the extraction carried out using n-hexane as solvent in a 5 L Soxlet extractor for 8 h. The oil extracted was stored in the refrigerator at 4°C for further analyses of its physico-chemical properties.
Control method: The traditional extraction procedure
The traditional extraction method of shea butter involved pounding the kernel in a mortar and pestle, roasting the pulverized kernel and milling to paste, followed by kneading of the paste; the oil that floats to the surface was scooped off, boiled and the extracted shea butter skimmed off to solidify.
Determination of the moisture content of the shea butter
Moisture is a chemical contaminant usually mixed with oil. Presence of moisture in oil affects the quality of the oil and significant amount of moisture in oil support microbial growth (Alirezali et al., 2011; Hee, 2011) and lipid oxidation leading to rancidity (Hee, 2011) thereby reducing the shelf life of the fat and its corresponding products. Conversely, low moisture content of shea butter is indicative of good quality (Olaniyan and Oje, 2007). Difference in moisture content of shea butter can be attributed to shea vegetation (Quainoo et al., 2013), although the minimum moisture content of shea butter is 0.05% but can go as high as 2.0% (West African Regional Standards, 2006).
The moisture content was carried out according to Abdulai et al. (2015), with slight changes to the procedure. Five grams (5 g) of enzyme-assisted traditionally extracted shea butter was weighed and dried in an oven at 100°C. After every 1 h, the sample was removed from the oven and placed in the desiccator for 30 min to cool. It was then removed and weighed. The percentage moisture in the butter was then calculated from:
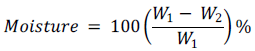
Where, W1 = original weight of sample before drying (g) and W2 = weight of sample after drying (g).
Determination of insoluble impurities
Insoluble impurities refer to dirt and other foreign materials in shea butter (Hamilton et al., 1986; Hee, 2011). It has been reported that some of these materials are bonded to the butter via the machinery employed in the extraction of the butter. Insoluble impurities may also make their way into the butter through physical contact of the butter with the soil, water, ground as well as packaging materials. The amount of insoluble impurities is identified as another important quality parameter which determines shea butter deterioration since metals can catalyse the oxidation of shea butter and thus decreases its market value (Hee, 2011).
The insoluble impurities were determined using the IUPAC 2.604 method as described in Paquot et al. (1987). By this method, 10 ml capacity gastight syringe was filled with 4 ml of melted shea butter sample and the syringe was then weighed. A filter and the filter paper were weighed and then placed in a filter holder and attached to the tip of the syringe. Pressure was applied on the syringe to cause the Shea butter sample to pass through the filter. The filter and the filter paper in the holder were washed with 30 ml of hexane to remove the shea butter from the device. The filter paper was then dried in an oven at the temperature of 100°C for 10 min to evaporate all solvent and then weighed. The insoluble impurities that remained in the filter paper were then calculated from the formula below:
Where, w is the weight of the shea butter, m1 is the mass of the filter paper and thefilter, m2 is the mass of the filter paper with the insoluble impurities and the filter.
Determination of free fatty acid
Free fatty acids (FFA) by definition are the fatty acids present in oil or fat which has not been neutralized (Guy, 2009) or just unattached fatty acids present in a fat (Sapna and Nirmali, 2009). FFAs are related to their acid values. The acid value is a parameter expressed as the number of milligrams of potassium hydroxide required to neutralize the free fatty acids contained in one gram of fat or oil (Kardash and Tur‟yan, 2004) and it is twice the FFA of a fat. AV is directly proportional to FFA (Roger et al., 2010), and thus the lower the acid value of oil, the fewer FFA it contains.
Low acid value suggests low levels of hydrolytic activities in the oils (Ikya et al., 2013), and this reduce the exposure of the fat to the phenomenon of rancidification (Roger et al., 2010). Thus, the degree of rancidity (index for butter freshness) depends upon the acid value (Khan et al., 2006). FFA determination means the establishment of the level of rancidity, quality and edibility of shea butter (Onyeike and Oguike, 2003) and, that is, the nutritional potential of shea butter rely in upon the quantity of FFA content the value of which should fall within the threshold limits of 0 0.6 and 10% (Codex Alimentairus Commission, 1993).
The FFA was determined according to Akpan et al. (2006), as described in Abdulai et al. (2015).Two grams (2 g) of the shea butter sample was accurately weighed into a 250 ml conical flask with a glass stopper. Fifty (50 ml) of hot neutralized isopropyl alcohol (IPA) solution was added. This was followed with the addition of few drop of phenolphthalein. The content was titrated against 0.1 N NaOH with vigorousshaking until a permanent pink colour was obtained. The percentage FFA wascalculated as follows:
Where, WB = weight of shea butter.
Peroxide value determination
Peroxide value (PV) is an indicator for stability and level of deterioration of shea butter and it measures the milliequivalents of oxygen or hydroperoxides in 1 g of fat or oil (Ikya et al., 2013). It is a valuable measure of oil quality as it serves as an indicator of degradation of the long fatty acid chains through auto-oxidation into peroxides that can later break down into other chemicals including foul-smelling ketones and aldehydes.The parameter is said to be inversely related to the stability of oil or fat. The oil with higher stability is less exposed to rancidity. High peroxide value say (PV>10 meq kg-1) is associated with the development of rancidity in fats and oils, which eventually limits their use in the food industry (Shahidi, 2005). Peroxide value is said to be the most common determinant of lipid oxidation (Shahidi, 2005). Hydroperoxides under normal condition is remarked to have no flavour or odour of their own, they are however unstable and usually break down rapidly to other products such as aldehydes that have a strong, disagreeable flavor and scent
Peroxide value was determined according to Abdulai et al. (2015). Five grams (5 g) of the shea butter sample was accurately weighed into 250 ml conical flask. 30 ml of solvent mixture of acetic acid/cyclo-hexane in the ratio of 3:2 respectively was added and the flask shaken to completely dissolve the butter. 0.5 ml of freshly prepared saturated potassium iodide (KI) solution was added to the solution and allowed to stand for 1min. Thereafter, 30 ml distilled water was added followed by 0.5 ml starch solution. The content was titrated with 0.01N Na2S2O3 solution until the dark-blue colour disappeared. Blank titration was also conducted alongside the sample test. The peroxide was calculated from the relation:
Where, V1 is the volume of Na2S2O3 solution in ml for determination of test sample, V2 is the volume of Na2S2O3 solution in ml for determination of blank and M is the mass of shea butter sample.
Apart from the method of enzyme assisted traditional extraction of shea butter, two other methods were employed concurrently as positive controls for the extraction of shea butter; the traditional and the solvent extraction techniques. The qualities of shea butter from the three methods were thus examined and compared and conclusions were drawn in relation to well-developed and documented shea butter quality standards for unrefined shea butter.
Moisture content of substrates prior to extraction
Although all three substrates showed relatively low moisture contents, the ROSK sample showed the lowest moisture content of 5.71%, RASK with 7.22% moisture content and the highest moisture levels was observed with SKEP at 10.32%. The traditional floor drying after 14 days of sun-drying produced moisture content of values ranging from 7 to 7.5% (Aculey et al., 2012) and this is consistent with the moisture content of the raw shea kernels (7.22%) originally processed by the traditional sun-drying method. Further roasting/heating would allow more dehydration of the kernel and so it is not surprising that moisture content of the roasted kernels plunged down to 5.71 %.
Moisture content of shea butter
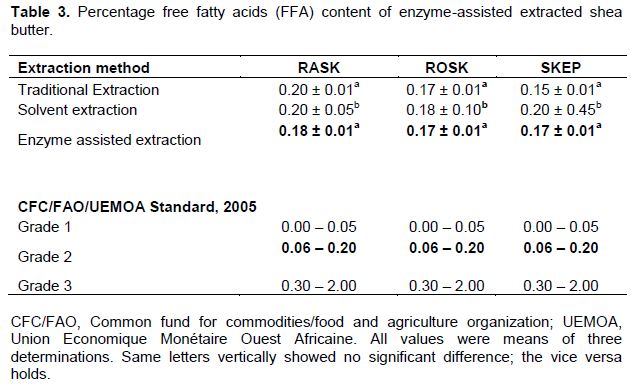
The moisture content of all the three types of shea nut butter ranged from 0.15 to 0.20%. Moisture content of enzyme assisted shea butter differed by the pretreatment – moisture content of shea butter from the roasted kernels differed from that of shea butter from the raw kernels (Table 1). The average moisture content from enzyme assisted shea butter was 0.17%. Other researchers (Asuquo et al., 2006; Hee, 2011; Abdulai et al., 2015) that used non-enzyme assisted methods measured moisture levels of shea butter at 10 13 and 5.39% respectively. The lowest butter moisture content (0.17%) was found with this research but that still falls short of the required standards recommended by the West African Regional Standards and the Global Shea Alliance (GSA) for “Grade” 1 (≤0.05%) shea butter. The current enzyme-assisted extracted shea butter is of “Grade 2” (˃0.05 to 0.2%) (Table 2); the reason for its limited use in the cosmetic and saponification industries otherwise there must be further refinement of the butter. With 0.17 % moisture content the shea butter is susceptible to microbial attack leading to the degradation of the shea butter since the fatty acids will remain the major carbon source for the microorganisms. Thus, the use of such butters in the pharmaceuticals industry will affect the stability and shelf life of the products. This may not be the same in cosmetic making and saponification processes since the shea butter is subjected to further heating to dryness.
Free fatty acids (FFA)
FFA was highest (4.13%) in shea butter extracted from ROSK by solvent extraction method (Table 3) and this was expected. Chemicals unlike enzymes are harsh and unspecific in their attack of chemical bonds following the principle of dissolution and this can result in the formation of more saturated fats or FFAs. In addition, FFA increases with increasing temperature (Arena et al., 2013). The FFA was lowest in shea butter from raw kernels (RASK) by enzyme-assisted extraction and lowest in the butter from RASK from the other methods of extraction: 1.85% by the traditional method and 3.85% by solvent extraction method (Table 3). Roasted kernels (Table 3) produced higher FFA levels (2.09, 4.13 and 2.71%) in the butter from traditional, solvent and enzymatic methods respectively. The FFA values from other studies (Adomako, 1997; Nkouam et al., 2007; Agyente-Badu, 2010; Hee, 2011; Abdulai et al., 2015) using non-enzymatic methods have been relatively high (6.80, 21.20, 6.80, 5.01, 5.30%) respectively except for Honfo et al. (2013) who recorded a 0% FFA free shea butter from shea kernels pretreated with smoke rather than boiling that yielded butter with 6% FFA. The range of FFA of shea butter by enzymatic extraction (1.6 – 2.7%) from the current study qualifies it as “Grade 2” (1.10 – 3.00%).
Gezahegn et al. (2016), using the mechanical press at 70áµ’C measured the FFA levels of shea butter with a minimum value of 0.62% to a maximum of 2.23%. In addition to temperature considerations, the incidence of FFA depends on the time between harvesting and boiling since the activity of fruit lipase and microorganisms increase FFA levels (Abdul-Mumeen et al., 2013; Nitiema-Yefanova et al., 2012). But on the contrary, microbial lipases are said to be very effective for triglyceride hydrolysis at the α-positions to produce monoglycerides more rapidly than at the β-positions (Pablo et al., 1974) and thus lipases from S. epidermidis, for instance, do not produce FFA’s (Abdul-Mumeen et al., 2013).
Peroxide value (PV)
It was observed that the peroxide value significantly differed (P ≤ 0.05) among the three different shea butter samples (Table 4). The least PV value of 3.67 mEq was observed in fat from the RASK by enzyme assisted extraction and the highest PV value (11.46) mEq in SKEP by solvent extraction. The PV values between the fat obtained by the traditional (4.86) and the enzyme-assisted (3.67) methods differed significantly but only with the RASK (Table 3). The PVs of fat by solvent extraction method was generally high (9.95 – 11.46 meq/kg) in all three substrates. It was reported that PVs are likely influenced by high moisture (Akingbala et al., 2006) and insoluble impurities levels and this trend was observed in the findings (Tables 2 and 5). Another factor that can influence the PV levels is the quality of the shea kernels used, as bad kernels can increase insoluble impurities levels of fats extracted and this could trigger auto-oxidation processes in the fat.
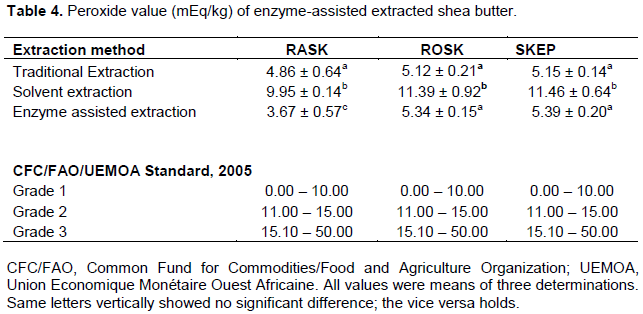
Tano-Debrah and Ohta (1995) obtained 11.18 mEq PV from shea butter extracted from roasted kernels pretreated with enzymes and that was quiet high compared to the present findings of 5.34 mEq. There could be other causal factors affecting the PV rather than just the method of extraction. The shea nut kernels were subjected to thorough cleaning, sorting and separation to obtain the best quality ones for the extraction of the fat and this could have impacted the insoluble impurities which have consequential effects on the PV. Although insoluble impurities in shea butter which is normally referred to dirt and other foreign materials (Hamilton et al., 1986; Hee, 2011), lipoxygenase catalyzed oxidation can increase the PV mainly due to residual water (Akingbala et al., 2007). Other non-enzymatic methods yielded different levels of PVs. Asuquo et al. (2006), Hee (2011), Abdulai et al. (2015) using the traditional method, respectively obtained 14.20, 12.03 and 8.06 mEq PVs of shea nut fat. About 3.55 and 3.70 mEq PV of shea butter by mechanical press were obtained by Francis (2009) and Gezahegn (2014) respectively. By solvent extraction methods, 2.20 (Francis, 2009), 9.65 (Tano-Debrah and Ohta, 1994) and 2.50 mEq (Okullo, 2010) PVs have been recorded.
Insoluble impurities
Among all the quality parameters of shea butter, insoluble impurities in the present study (0.02%) sought to be emanated from a common source (Table 5). The insoluble impurities were not significantly different (P ≥ 0.05) across methods and among the various levels of kernel pretreatment. The traditional extraction process in its original sense has several offshoots to contaminating the shea butter: the level of care for the kernel processing, the water used for the butter extraction process, the containers involved in the process, etc., all contribute to the insoluble impurities in the final product – shea butter. Hee (2011) and Abdulai et al. (2015), using the traditional process obtained 0.14 and 0.13% of insoluble impurities respectively but this is comparable to the values measured (0.03 and 0.02) during the current research.
The Common Fund for Commodities (CFC) in collaboration with the Food and Agriculture Organization (FAO) has developed shea butter standards now adopted by the Union Economique Monétaire Ouest Africaine (UEMOA). The standards categorizes shea butter into three: Grade 1, butter recommended as suitable for the cosmetic and pharmaceutical industries and for direct consumption; Grade 2 shea butter is deemed suitable for the food industries and for manufacturing confectionaries, chocolate, edible oil and for making margarine; Grade 3 shea butter is recommended for the saponification industry although further refinement could qualify it for direct consumption.
The use of enzymes such as lipases, pectinases and cellulases in the extraction of shea butter generally results in the production of fat with enhanced properties (Table 6). The enzymatic extraction of shea butter enhances its quality as Grade 1 material based on the levels of its impurities and peroxide values irrespective of the level of pretreatment (Tables 2, 3 and 4). On the basis of moisture content and FFA levels, enzyme-assisted extracted shea butter will fall under Grade 2 (Tables 2, 3 and 4).