Full Length Research Paper
ABSTRACT
To our knowledge, no research study has been carried out on the effects of ascorbic acid (ASA), 5-Aminolevulinic acid (ALA) and Nano selenium (N-Se) on the cytological parameters of pea seedlings under salinity stress. Salinity treatment (60 and 120 mM NaCl) was applied. Two concentrations of ASA (50 and 100 ppm), ALA (25 and 50 ppm), and N-Se (10 and 20 ppm), respectively were used individually and in combination with NaCl (60 and 120 mM). Modifications in shoot length, number of leaves, leaf area, chromosomal aberrations and mitotic index were determined. Salinity treatment (120 mM) caused the highest reduction in shoot length, leaf area and mitotic index. A significant increase of chromosomal abnormalities percentage (%) was detected in salinity treatments compared with control. ASA (100 ppm), ALA (50 ppm) and N-Se (10 ppm) treatments significantly reduced the damaging effect of salinity stress on growth attributes, mitotic index and chromosomal abnormalities percentage (%) and improved seedlings’ performance. These treatments can be recommended for the improvement of pea plants’ productivity under salt stress.
Key words: Ascorbic acid, 5-aminolevulinic acid, nano selenium, salt stress, mitosis, chromosomal aberrations, Pissum sativum L.
INTRODUCTION
Salt stress adversely affects the morphological, physiological and biochemical responses of plant species (Nazar et al., 2011). Several researchers found that the chlorophyllian pigments were reduced with an increase in salinity level. This may be due to the disruption of the fine structure of chloroplasts and pigment-protein complex or chlorophyll stability, which can result in chlorophyll oxidation (Saha et al., 2010; Helaly et al., 2016; Elsheery et al., 2020c) and disturb plant growth and development (Sairam and Tyagi, 2004). Tang et al. (2017b) established that salinity inhibits plant growth, reduces yield in many crop plants and affects their commercial value (Helaly et al., 2016; Elsheery et al., 2020c). So, salinity stress inhibits growth of basil plants by decreasing a significant number of leaves/plant and plant height (Khan et al., 2009; Nassar et al., 2019). Also, the retardant effects of salinity stress on growth, physiological aspects and productivity were also recorded on other different plants species; for instance, Reda (2007) on Senna occidentalis, Dawood et al. (2014) on Faba bean, Bargaz et al. (2016) on Phaseolus vulgaris, Nassar et al. (2016) on Leucaena and Elsheery et al. (2020b) on mango. There are many ways to improve salinity tolerance in plants such as using of biofertilizer and amino acids (Helaly et al., 2016) and grafting in vegetable crops (Elsheery et al., 2020a; Helaly et al., 2016; Al-Mayahi, 2016). This study was carried out to investigate the effects of ascorbic acid (ASA) under salinity stress on growth of pea plant. Some biochemical constituents that can promote growth and increase productivity of many species of plants grown under normal or abiotic stress conditions are highly recommended (Sharma et al., 2019). Ascorbic acid (ASA) is a small water soluble antioxidant molecule which acts as an essential substrate in the cyclic pathway of enzymatic detoxification of hydrogen peroxide. Ascorbic acid (ASA) is a naturalist product that acts as an antioxidant and enzyme and also improves cofactor. It engages in a variety of procedures. It correlates with chloroplasts in the oxidative stress of photosynthesis (Latif et al., 2016). Furthermore, ASA has a number of roles in protein modification and cell division in plant cells (Hussein et al., 2019). Nowadays, it plays an essential role in a series of physiological processes such as cofactor of key enzyme, plant defense against oxidization, growth, development, cell division, cell extension, senescence and counteracts the deleterious effects of biotic and abiotic stresses (Zhang and Sonnewald, 2017). Therefore, it is chosen to be one of the substances of the subject of our present study.
5-Aminolevulinic acid (ALA) is a type of non-protein amino acid that supports plant stress tolerance. However, the underlying physiological and biochemical mechanisms are not entirely understood (Anwar et al., 2020). ALA is found in all plants and animals. 5-aminolevulinic acid (ALA) is and a key precursor for the biosynthesis of porphyrins such as chlorophyll, heme and plant hormones. In addition, it has newly been reported that ALA regulates the expression level of fructose-1, 6-bisphosphatase (FBP), triose-3-phosphate isomerase (TPI), and ribulose-1, 5-bisphosphate carboxylase/ oxygenase small subunit (RBCS), which activate the Calvin cycle of photosynthesis under drought stress (Liu et al., 2016). It was found that, ALA is one of plant growth regulators (PGRs) and mitigates salinity stress effect in germinating seeds and ameliorates seedling growth. Foliar application of 5-aminolevulinic acid at low concentrations has been shown to promote salt tolerance in a lot of plants (Tang et al., 2017a). On the other hand, ALA is involved in the chlorophyll biosynthesis pathway under salt stress conditions (Wu et al., 2011) and motivates antioxidant enzyme efficiency and accumulation of endogenous hormone under many stress factors such as low-temperature in cucumber seedlings (Anwar et al., 2018). Under drought stress, spray application of ALA up-regulated the chlorophyll fluorescence indexes in oilseed rape (Brassica napus L.) (Liu et al., 2014) and gas exchange indexes, such as net photosynthetic average (Pn), stomatal behavior (gs), intercellular CO2 concentration (Ci) and the rate of transpiration (Tr), which were adversely influenced by abiotic stress (Wu et al., 2018). It is also reported that foliar application of ALA may confer plant tolerance to diverse abiotic stresses, such as chilling, high temperature, salinity, drought, weak light, and heavy metals (Wu et al., 2019a). Previous studies demonstrated that ALA encourages abiotic stress tolerance by activation of numerous types of transcription factors, signal transduction, and chlorophyll and carbohydrate biosynthesis (Nishihara et al., 2003; Anwar et al., 2020). These results submit that ALA can broadly minimize the harmful effects of environmental stress. Increasing attention has been paid to the beneficial impacts of many nanoparticles (NPs) used in low doses on diverse crops (Jampílek and Kráľová, 2017; Rastogi et al., 2019; Kumar et al., 2020; Elsheery et al., 2020c). A lot of researchers like Sonkaria et al. (2012) and Prasad et al. (2014) established that, using of NPs can promote plant growth, warrant food goodness and decrease waste. Nano-Selenium (N-Se) as Nano fertilizer has been recently used in the field (Shang et al., 2019; Elsheery et al., 2020a; Elsheery et al., 2020b). There is less documented information on the biological effects of N-Se and its application (Chau et al., 2007; Cushen et al., 2012). Bhattacharjee et al. (2014) and Kamle et al. (2020) suggest that N-Se plays a role as a reactive oxygen species (ROS) scavenger in plants under stress conditions. So, the purpose of our study was: To evaluate the (ASA, ALA and N-Se) morphological and cytological effect of application of our treatments (Soaked and foliar) on pea plants under salinity stress using hydroponic methods.
MATERIALS AND METHODS
RESULTS AND DISCUSSION
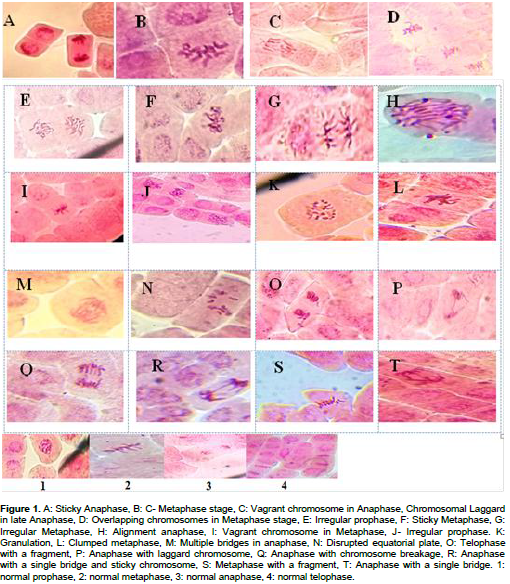
CONCLUSIONS
ACKNOWLEDGEMENTS
REFERENCES
|
Abdul Qados MSA (2011). Effect of salt stress on plant growth and metabolism of bean plant Vicia faba (L.). Journal of the Saudi Society of Agricultural Sciences 10:7-15. |
|
|
Acosta-Motos JR, Penella C, Hernández JA, Díaz-Vivancos P, Sánchez-Blanco M J, Navarro JM, Gómez-Bellot MJ, Barba-Espín G (2020). Towards a Sustainable |
|
|
Agriculture: Strategies Involving phytoprotectants against Salt Stress. Agronomy 10:1-32. |
|
|
Ali RM (2000). Role of putrescine in salt tolerance of Atropa belladonna plant. Plant Science 152:173-179. |
|
|
Al-Kaisy W, Sahar A, Mahadi F (2018). Response of pea (Pisum sativum L.) to foliar application of ABA and vitamin C and interaction of them on some physiological characters of plant. Al-Mustansiriyah Journal of Science 29:32-37. |
|
|
Al-Karaki GN (2001). Germination, sodium and potassium concentrations of barley seeds as influenced by salinity. Journal of Plant Nutrition 24:511-522. |
|
|
Al-Mayahi AMW (2016). Influence of salicylic acid (SA) and ascorbic acid (ASA) on in vitro propagation and salt tolerance of date palm (Phoenix dactylifera L.) cv. 'Ner¬sy'. Australian Journal of Crop Science 10(7):969-976. |
|
|
Anwar A, Wang J, Yu X, He Ch, Li Y (2020). Substrate Application of 5- aminolevulinic acid enhanced low-temperature and weak-light stress tolerance in cucumber (Cucumis sativus L.). Agronomy 10:1-12. |
|
|
Anwar A, Yan Y, Liu Y, Li Y, Yu X (2018). 5-aminolevulinic acid improves nutrient uptake and endogenous hormone accumulation, enhancing low-temperature stress tolerance in cucumbers. International Journal of Molecular Science 19:33-49. |
|
|
Autifi M, Mohamed W, Abdul Haye W, Elbaz K (2018). The possible protective role of vitamin C against toxicity induced by lead acetate in liver and spleen of adult albino rats (light and electron microscopic study). The Egyptian Journal of Hospital Medicine 73:7650-7658. |
|
|
Barakat H (2003). Interactive effects of salinity and certain vitamins on gene expression and cell division. International Journal of Agriculture and Biology 5:219-225. |
|
|
Bargaz A, Nassar RMA, Rady MM, Gaballah MS, Thompson SM, Brestic M, Schmidhalter U, Abdelhamid MT (2016). Improved salinity tolerance by phosphorus fertilizer in two Phaseolus vulgaris recombinant inbred lines contrasting in their p-efficiency. Journal of Agronomy and Crop Science 202:497-507. |
|
|
Bhattacharjee A, Basu A, Ghosh P, Biswas J, Bhattacharya S (2014). Protective effect of Selenium nanoparticle against cyclophosphamide induced hepatotoxicity and genotoxicity in Swiss albino mice. Journal of Biomaterials Applications 29:303-317. |
|
|
Bronzetti G, Cini M, Andreoli E, Caltavuturo L, Panunzio M (2001). Protective effects of vitamins and selenium compounds in yeast. Croce CD. PubMed 496:105-115. |
|
|
ÇavuÅŸoÄŸlu K, Cadıl S, ÇavuÅŸoÄŸlu D (2017). Role of potassium nitrate (KNO3) in alleviation of detrimental effects of salt stress on some physiological and cytogenetical parameters in Allium cepa L. Cytologia 82:279-286. |
|
|
ÇavuÅŸoÄŸlu K, Kaya F, Kılıç S (2013). Effects of boric acid pretreatment on the seed germination, seedling growth and leaf anatomy of barley under saline conditions. Journal Food Agriculture and Environment 11:376-380. |
|
|
ÇavuÅŸoÄŸlu K, Kılıç S, Kabar K (2007). Some morphological and anatomical observations during alleviation of salinity (NaCl) stress on seed germination and seedling growth of barley by polyamines. Acta Physiologiae Plantarum 29:551-557. |
|
|
Chau CF, Wu SH, Yen GC (2007). The development of regulations for food nanotechnology. Trends in Food Science and Technology 18:269-280. |
|
|
Colla G, RouphaelY, Cardarell M, Massa D, Salerno A, Rea, E (2006a). Yield, fruit quality and mineral composition of grafted melon plants grown under saline conditions. Horticulture Science and Biotechnology 81:146-152. |
|
|
Colla G, RouphaelY, Jawad R, Cardarell M, Rea E (2006b). Effect of salinity on yield, fruit quality, leaf gas exchange and mineral composition of grafted watermelon plants. Horticulture Science and Biotechnology 41:622-627. |
|
|
Cushen M, Kerry J, Morris M, Cruz-Romero M, Cummins E (2012). Nanotechnologies in the food industry-Recent developments, risks and regulation. Trends in Food Science and Technology 24:30-46. |
|
|
Darlington C (1976). La cour. L.R, The Handling of Chromosomes, George Alien and Unwin, London P. 182. |
|
|
Dawood MG, Taie HAA, Nassar RMA, Abdel hamid MT, Schmidhalter U (2014). The changes induced in the physiological, biochemical and anatomical characteristics of Vicia faba by the exogenous application of proline under seawater stress. South African Journal of Botany 93:54-63. |
|
|
Desoky EM, Merwad AM, Elrys AS (2017). Response of pea plants to natural bio-stimulants differences and commonalities of plant responses to single and combined stresses, under soil salinity stress. American Journal and Plant Physiology 12:28-37. |
|
|
Ekanayake LJ, Thavarajah D, Vial E, Schatz B, McGee R, Thavarajah P (2015). Selenium fertilization on lentil (Lens culinaris Medikus) grain yield, seed selenium concentration and antioxidant activity. Field Crops Research 177:9-14. |
|
|
Elkelish A, Qari SH, Mazrou YSA, Abdelaal KAA, Hafez YM, Abu-Elsaoud AM, Batiha GE, El-Esawi MA, El Nahhas N (2020). Exogenous ascorbic acid induced chilling tolerance in tomato plants through modulating metabolism, osmolytes, antioxidants and transcriptional regulation of catalase and heat shock proteins. Plants 9:1-27. |
|
|
Elsheery N, Sunoj VSJ, Wen Y, Zhu JJ, Muralidharan G, Cao KF (2020a). Foliar application of nanoparticles mitigates the chilling effect on photosynthesis and photoprotection in sugarcane. Plant Physiology and Biochemistry 149:50-60. |
|
|
Elsheery N, Helaly M, El-Hoseiny H, Alam-Eldein Sh (2020b). Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy 10:953-975. |
|
|
Elsheery N, Helaly M, Omar S, John S, Zabochnicka-Swiatek M, Kalaji H, Rastogi A (2020c). Physiological and molecular mechanisms of salinity tolerance in grafted cucumber. South African Journal of Botany 130:90-102. |
|
|
Gama PBS, Inanaga S, Tanaka K, Nakazawa R (2007). Physiological response of common bean (Phaseolus vulgaris L.) seedlings to salinity stress. African Journal of Biotechnology 6:79-88. |
|
|
Germ M, Kreft I, Stibilj V, Urbanc-BerÄiÄ O (2007). Combined effects of selenium and drought on photosynthesis and mitochondrial respiration in potato. Plant Physiology and Biochemistry 45:162-167. |
|
|
Ghezal N, Rinez I, Sbai H, Saad I, Farooq M, Rinez A, Zribi I, Haouala R (2016). Improvement of Pisum sativum salt stress tolerance by bio-priming their seeds using Typha angustifolia leaves aqueous extract. South African Journal of Botany 105:240-250. |
|
|
Ghoulam C, Fares K (2001). Effect of salinity on seed germination and early seedling growth of sugar beet (Beta vulgaris L.). Seed Science Technology 29:357-364. |
|
|
Hanafy MS, El-Banna A, Schumacher HM, Jacobsen HJ, Hassan FS (2013). Enhanced tolerance to drought and salt stresses in transgenic faba bean (Vicia faba L.) plants by heterologous expression of the PR10a gene from potato. Plant Cell Reports 32:663-674. |
|
|
Helaly M, El-Hosieny H, Elsheery N, Kalaji H (2016). Effect of biofertilizers and putrescine amine on the physiological features and productivity of date palm (Phoenix dactylifera L.) grown on reclaimed-salinized soil. Trees 30:1149-1161. |
|
|
Hoagland DR, Arnon DI (1950). The water culture method for growing plants without soil. California Agricultural Experimental Station Circular. University of California, Berkeley 347:1-32. |
|
|
Hoda Q, Bose S, Sinha S (1991). Vitamin C mediated minimization of malathion and rogor induced mito-inhibition and clastogeny. Cytologia 56:89-97. |
|
|
Hopkins WG (1995). Introduction to Plant Physiology. New York: John Wiley pp. 72-73. |
|
|
Hotta Y, Tanaka T, Takaoka H, Takeuchi Y, Konnai M (1997). New physiological effects of 5-aminolevulinic acid in plants: the increase of photosynthesis, chlorophyll content, and plant growth. Bioscience, Biotechnology and Biochemistry 61:2025-2028. |
|
|
Hussein MM, Abd El-Khader A, El-Faham SY (2019). Mineral status and lupine yield responses to ascorbic acid spraying and irrigation by diluted sea water. Asian Journal of Biology 8:1-13. |
|
|
Jampílek J, Kráľová K (2017). Nanomaterials for delivery of nutrients and growth-promoting compounds to plants. Nanotechnology: An Agricultural Paradigm 9:177-226. |
|
|
Kamle M, Devi Sh, Mahato DK, Soni R, Kumar P, Tripathi V (2020). Nanotechnological interventions for plant health improvement and sustainable agriculture. Recent Patents on Food, Nutrition and Agriculture 10:168-181. |
|
|
Kamran M, Parveen A, Ahmar S, Malik Z, Hussain S, Chattha M, Saleem M, Adil M, Heidari P, Chen J (2020). An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms and amelioration through selenium supplementation. International Journal of Molecular Sciences 21:1-27. |
|
|
Khan NA, Nazar R, Anjum NA (2009). Growth, photosynthesis and antioxidant metabolism in mustard (Brassica juncea L.) cultivar differing in ATP-sulfurylase activity under salinity stress. Scientia Horticulturae 122:455-460. |
|
|
Kumar KA, Sugunamma V, Sandeep N (2020). Influence of viscous dissipation on MHD flow of micropolar fluid over a slendering stretching surface with modified heat flux model. Journal of Thermal Analysis and Calorimetry 139:3661-3674. |
|
|
Latif M, Akram, NA, Ashraf M (2016). Regulation of some biochemical attributes in drought-stressed cauliflower (Brassica oleracea L.) by seed pre-treatment with ascorbic acid. Journal Horticulture Science Biotechnology 91:129-137. |
|
|
Liu D, Hu L, Ali B, Yang A, Wan G, Xu L, Zhou W (2016). Influence of 5-aminolevulinic acid on photosynthetically related parameters and gene expression in Brassica napus L. under drought stress. Soil Science and Plant Nutrient 62:254-262. |
|
|
Liu L, Nguyen NT, Ueda A, Saneoka H (2014). Effects of 5-aminolevulinic acid on swiss chard (Beta vulgaris L. subsp. cicla) seedling growth under saline conditions. Frontiers in Plant Science 74:219-228. |
|
|
McCue KF, Hanson AD (1990). Drought and salt tolerance: Towards understanding and application. Trends Biotechnology 8:358-362. |
|
|
Memon SA, Hou X, Wang LJ (2010). Morphological analysis of salt stress response of pak Choi. EJEAFCHe 9:248-254. |
|
|
Naeem MS, Rasheed M, Liu D, Jin ZL, Ming DF, Yoneyama K, Takeuchi Y, Zhou WJ (2011). Role of 5-aminolevulinic acid on growth, photosynthetic parameters and antioxidant enzyme activity in NaCl-stressed Isatis indigotica. Fort. Acta Physiologiae Plantarum 518:517-528. |
|
|
Naeem MS, Warusawitharana H, Liu H, Liu D, Ahmad R, Waraich EA (2012). 5-Aminolevulinic acid alleviates the salinity-induced changes in Brassica napus as revealed by the ultrastructural study of chloroplast. Plant Physiololgy and Biochemistry 57:84-92. |
|
|
Nassar MA, Azoz DN, Wessam S, Serag El-Din M (2019). Improved growth and productivity of basil plants grown under salinity stress by foliar application with ascorbic acid. Middle East Journal of Agriculture 8:211-225. |
|
|
Nassar R, Nermeen T, Reda F (2016). Active yeast extract counteracts the harmful effects of salinity stress on the growth of leucaena plant. Scienta Horticulturae 201:61-67. |
|
|
Nazar R, Iqbal N, Syeed S, Khan NA (2011). Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mung bean cultivars. Journal of Plant Physiology 168:807-815. |
|
|
Nishihara E, Kondo K, Masud Parvez M, Takahashi K, Watanabe K, Tanaka K (2003). Role of 5-aminolevulinic acid (ALA) on active oxygen-scavenging system in NaCl-treated spinach (Spinacia oleracea). Plant Physiology 160:1085-1091. |
|
|
Prakash L, Dutt M, Prathapasenan G (1988). NaCl alters contents of nucleic acids, proteins, polyamines and the activity of agmatine deiminase during germination and seedling growth of rice (Oryza sativa L.). Australia and Plant Physiology 15:769-776. |
|
|
Prasad R, Kumar V, Prasad KS (2014). Nanotechnology in sustainable agriculture: Present concerns and future aspect. African Journal of Biotechnology 13:705-713. |
|
|
Radić S, Pavlica M, Babić M, Pevalek-Kozlina B (2009). Cytogenetic effects of osmotic stress on the root meristem cells of Centaurea ragusina L. Environmental and Experimental Botany 54:213-218. |
|
|
Rafaqat A, Ali G, Islam F, Muhammad F, Theodore M, Zhou M (2015). Physiological and molecular analyses of black and yellow seeded Brassica napus regulated by 5-aminolivulinic acid under chromium stress. Journal of Plant Physiology and Biochemistry 94:130-143. |
|
|
Rastogi D, Kumar T, Saurabh Y, Chauhan D, ŽivÄák M, Ghorbanpour M, El-Sheery N, Brestic M (2019). Application of silicon nanoparticles in agriculture. PMC National Library of Medicine National Institutes of Health 9:90-102. |
|
|
Reda M (2007). Morphological, anatomical and physiological studies on Senna occidentalis (L.) Link plants grown under stress of different levels of salinity in irrigation water. Journal Agriculture Science, Mansoura University 32:8301-8314. |
|
|
Saha P, Chatterjee P, Biswas AK (2010). NaCl pre-treatment alleviates salt stress by enhancement of antioxidant defense system and osmolyte accumulation in mungbean (Vigna radiata L. Wilczek). Indian Journal of Experimental Biology 48:593-600. |
|
|
Sairam RK, Tyagi A (2004). Physiology and molecular biology of salinity stress tolerance in plants. Current Science 86:407-421. |
|
|
Sajid M, Mustafa A, Niamat B, Ahmad Z, Yaseen M, Kamran M, Rafique M, Ahmar S, Chen J (2020). Alleviation of salinity-induced oxidative stress, improvement in growth, physiology and mineral nutrition of canola (Brassica napus L.) through calcium-fortified composted animal manure. Sustainability 12:1-17. |
|
|
Shahzad H, Ullah S, Iqbal M, Bilal HM, Shah GM, Ahmad S, Zakir A, Ditta A, Farooqi MA (2020). Exogenous ascorbic acid induced chilling tolerance in tomato plants through modulating metabolism, osmolytes, antioxidants, and transcriptional regulation of catalase and heat shock proteins. Plants 9:1-21. |
|
|
Shalata A, Mittova V, Volokita M, Guy M, Tal M (2001). Response of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii to salt-dependent oxidative stress: the root antioxidative system. Physiology Plant 112:487-494. |
|
|
Shang Y, Hasan K, Golam J, Li M, Yin H, Zhou J (2019). Applications of nanotechnology in plant growth and crop protection. Molecules 24:1-24. |
|
|
Sharma A, Shahzad B, Kumar V, Kohli SK, Sidhu GPS, Bali AS, Handa N, Kapoor D, Bhardwaj R, Zheng B (2019). Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 9:1-36. |
|
|
Shedeed Sh, Fawzy Z, El-Bassiony A (2018). Nano and mineral selenium foliar application effect on pea plants (Pisum sativum L.). Bioscience Research 15:645-654. |
|
|
Shi Y, Wang Y, Flowers T, Gong H (2013). Silicon decreases chloride transport in rice (Oryza sativa L.) in saline conditions. Journal of Plant Physiology 170:847-853. |
|
|
Sonkaria S, Ahn SH, Khare V (2012). Nanotechnology and its impact on food and nutrition. Recent Patents on Food, Nutrition and Agriculture 4:8-18. |
|
|
Tabur S, Demir K (2010a). Role of some growth regulators on cytogenetic activity of barley under salt stress. Plant Growth Regulators 60:99-104. |
|
|
Tabur S, Demir K (2010b). Protective roles of exogenous polyamines on chromosomal aberrations in Hordeum vulgare exposed to salinity. Biologia 65:947-953. |
|
|
Tabur S, Demir K (2009). Cytogenetic response of 24-epibrassinolide on the root meristem cells of barley seeds under salinity. Plant Growth Regulators 58:119-123. |
|
|
Taïbi F, Taïbi K, Belkhodja M (2013). Salinity effects on the physiological response of two bean genotypes Phaseolus vulgaris L. Arab Gulf Journal of Scientific Research 31:90-98. |
|
|
Tang XQ, Wang Y, Xiao YH (2017a). role of 5-aminolevulinic acid on growth, photosynthetic parameters and antioxidant enzyme activity in NaCl-stressed Isatis indigotica Fort. Russian Journal of Plant Physiology 64:198-206. |
|
|
Tang Y, Liu K, Zhang J, Li X, Xu K, Zhang Y (2017b). JcDREB2, a physic nut AP2/ERF gene, alters plant growth and salinity stress responses in transgenic rice. Frontiers in Plant Science 8:306-324. |
|
|
Watanabe K, Tanaka T, Kuramochi H, Takeuchi Y (2000). Improving salt tolerance of cotton seedling with 5-aminolevulinic acid. Plant Growth Regulators 32:97-101. |
|
|
Wu W, Elsheery N, Wei Q, Zhang L, Huang J (2011). Defective etioplasts observed in variegation mutants may reveal the light independent regulation of white/yellow sectors of Arabidopsis leaves. Journal of Integrative Plant Biology 53:846-857. |
|
|
Wu Y, Jin X, Liao W, Hu L, Dawuda M, Zhao X, Tang Z, Gong T, Yu J (2018). 5-aminolevulinic acid (ALA) alleviated salinity stress in cucumber seedlings by enhancing chlorophyll synthesis pathway. Frontiers in Plant Science 9:635-646. |
|
|
Wu J, Zhao Q, Wu G, Yuan H, Ma Y, Lin H, Pan L, Li S, Sun D (2019a). Comprehensive analysis of differentially expressed unigenes under NaCl stress in flax (Linum usitatissimum L.) using RNA-Seq. International Journal of Molecular Science 20:2-8. |
|
|
Wu Y, Liao W, Dawuda M, Hu L, Yu J (2019b). 5-Aminolevulinic acid (ALA) biosynthetic and metabolic pathways and its role in higher plants: A review. Plant Growth Regulators 87:357-374. |
|
|
Yu J, Zhao W, Tong W, He Q, Yoon M, Li F, Choi B, Heo E, Kim K, Park Y (2019). Genome-wide association study reveals candidate genes related to salt tolerance in rice (Oryza sativa) at the germination stage. International Journal of Molecular Science 19:31-45. |
|
|
Zedan A, Omar S (2019). Nano Selenium: Reduction of severe hazards of atrazine and promotion of changes in growth and gene expression patterns on Vicia faba seedlings. African Journal of Biotechnology 18:502-510. |
|
|
Zhang CP, He P, Wei PX, Du DD, Yu ZL (2011). Effect of exogenous 5- aminolevulinic acid on seed germination and antioxidase activities of Perilla frutescens seedlings under NaCl stress. Chinese Traditional and Herbal Drugs 42:1194-1200. |
|
|
Zhang H, Sonnewald U (2017). Differences and commonalities of plant responses to single and combined stresses. The Plant Journal 90:839-855. |
|
|
Zhang Y (2013). Biological role of ascorbate in plants (Chapter 2). Springer Briefs in Plant Science 7-18. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0