ABSTRACT
A critical factor in the in vitro propagation protocol via somatic embryogenesis of coffee is the survival and growth of plants under ex vitro conditions. The objective of this work was to determine the effect of VIUSID Agro™ during the conversion of somatic embryos of coffee cv. Red Caturra rojo-884 in semi-controlled culture conditions. Two solutions (0.5 and 0.8 mL L-1) of the biostimulant were evaluated on in vitro plant. Applications were done twice daily for the first three days and thereafter once a day from day 7 to 90 days after transplanting. Survival rate at 15 days and morpho-physiological characteristics at 30, 60 and 90 days after transplanting were evaluated. All treatments with VIUSID-Agro™ and the control resulted in 100% survival. The concentration of 0.5 mL L-1 of VIUSID-Agro™ registered the better results in morpho-physiological variables. These results constitute the first report of the use of VIUSID Agro™ on in vitro plant of coffee during acclimatization phase.
Key words: Ex vitro acclimatization, coffee, conversion, somatic embryos, VIUSID Agro™.
Coffee is one of the most important agricultural products. It ranks second in international trade after oil. As a crop, it covers approximately 10.2 million hectares in more than 80 countries, especially in the tropical and subtropical regions of Africa, Asia and Latin America. The economy of many coffee producing countries depends largely on the profits from this crop. More than 100 million people earn their income directly or indirectly from coffee cultivated areas (Argoti and Belalcazar, 2017). In Cuba, the cultivation of coffee is an important aspect to increase the income of the national economy by concept of grain exportation. There are about 160,000 ha destined for cultivation (MINAG, 2018).
The economic importance of phyto-stimulants has grown in recent years, with market factors and the growing predominance of a culture of sustainability in economic development being the two main phenomena that drive the production and consumption of plant stimulants in the world. Plant biostimulants based on natural materials have received considerable attention by both the scienti?c community and commercial enterprises especially in the last two and a half decades (Yakhin et al., 2017). In view of this situation, the search for biological alternatives is necessary to solve the problems of fertilization of agricultural crops of agro-economic interest (Yu et al., 2004; Montano et al., 2007; Alarcón et al., 2012). Therefore, organic fertilization and phyto-stimulants are becoming increasingly important not only because of the yields that are usually achieved, but also because of the economic nature of their application and their contribution to the preservation of the environment (Núñez et al., 2010).
Foliar fertilization is a method by which nutrients are supplied to the plants through the leaves, basically in aqueous solutions in order to complement the fertilization performed in the soil, or to correct specific deficiencies in the same period of crop development. Physiologically speaking, all nutrients can be absorbed via foliar with greater or lesser speed at different times. This is so that theoretically the complete nutrition of the plant could be satisfied via foliar, but in practice it is not possible because of the high cost of numerous applications that would be necessary in order to meet the total requirements (Peña et al., 2015).
VIUSID Agro™ is another of the formulations that are used as plant growth stimulants. This has the particularity that all its components are subjected to the molecular activation technique, a procedure that leads to a considerable increase in the biological action of the substance. In addition, the product has been shown not to affect the environment and the health of the population. VIUSID Agro™ is a product that essentially contains Ascophyllum nodosum (algae extract), Potassium Phosphate, Malic Acid, Glucosamine, Aspartic acid, Arginine, Glycine, Tryptophan, Ascorbic acid, Zinc sulfate, Calcium Pantothenate, Pyridoxine, Folic acid, Cyanocobalamin, Monoammonium Glycyrrhizinate, Sodium Benzoate and Potassium Sorbateamino (Catalysis, 2019).
Gomez-Kosky et al. (2020) were the first researchers to study the effects of VIUSID Agro™ with reported very good results during ex vitro acclimatization of sugarcane (Saccharum spp.) in vitro plants in Cuba. The objective of this research is to study the effects of VIUSID Agro™ on the conversion of coffee somatic embryos.
Plant materials
A total of 56 in vitro plants obtained by somatic embryogenesis of coffee (C. arabica L. cv. Red Caturra rojo-884) with 3 pairs of true leaves, 2.5–3.5 cm height and with radical development according to the protocol developed by Ortiz et al. (2017) were used by treatment. Before acclimatization, plants were washed with running water. They were subsequently placed in black polypropylene trays of 28 holes with a capacity of 200 cm3 each, with a substrate composed of compost from bagasse (remains of the sugarcane industry), to which zeolite was added in proportion of 3:1 (v/v).
Effect of VIUSID Agro™ on ex vitro acclimatization of in vitro plants
Two solutions of VIUSID Agro™ (Catalysis, Spain) (0.5 and 0.8 mL L-1) were prepared and applied on the leaves during the first 3 days after transplanting. These applications were done twice a day; at 9:00 am and at 4:00 pm. After one week in the ex vitro acclimatization phase, applications were reduced to a weekly basis up until 90 days under these conditions. These were carried out with a 16-L capacity backpack sprayer (Matabi, Spain), with flood nozzle (flood-jet) Lurmark AN 2.5, according to its technical parameters. In vitro plants from somatic embryos were used as control to which no applications of the biostimulant were made.
Survival rate, defined as the number of plants that survived, was assessed at 7 and 15 days. At 30, 60 and 90 days the height of the plants (cm) from the base to the apex, the number of leaves, length of the main root, and number of roots were evaluated. In addition, the fresh weight (gFW), the dry weight (gDW) and the total chlorophyll content (SPAD units) were evaluated as well.
Determination of fresh and dry weight
For the determination of fresh and dry weight, 10 plants from treatments were taken at 30, 60 and 90 days of cultivation in the shade house. The roots were washed with water to remove substrates. The fresh weight was determined with an analytical balance (SCALTEC, model SPD 54). Subsequently, for the determination of the dry weight, the plant material was dried in an oven (MERMERT) at a temperature of 70°C for 48 h and the weight was determined on an analytical balance (SCALTEC, model SPD 54).
Determination of total chlorophylls
The total chlorophyll content was determined using the SPAD-502 chlorophyll detector (Minolta, Japan). SPAD units are recorded through the chlorophyll meter that measures the relative concentration of chlorophyll by means of transmitted light through the leaf at 650 nm (photosynthetically active wavelength) and 940 nm (Piekielek et al., 1995).
Culture conditions
The trays with the in vitro plants were placed in a shade house, with black shade mesh (Saran) that allowed the light intensity to be reduced to 70%. Other environmental conditions in the shade house include an average daytime temperature of 27 ± 5°C, relative humidity of 70% and light intensity that ranged between 224 and 457 µmol m-2 s-1 measured with a LM 76 Light Meter luxmeter (Paris, France). An automated micro sprinkler irrigation system was used. The frequency of the irrigation was four times a day for two minutes.
Experimental design and statistical analysis
The experimental design used was completely randomized. The Shapiro-Wilk test and the homogeneity of variance by Levene were used in the analysis of normality of the variables. For the comparison between the means, a variance analysis (simple ANOVA) was applied and the difference between the means was determined by the Tukey test. The SPSS Statistical Package version 23.0 of 2015 for Windows was used. In all cases, the significant differences were established for p≤0.05. All experiments were carried out twice.
Effect of VIUSID Agro™ on ex vitro acclimatization of in vitro plants
In vitro plants in both concentrations of VIUSID-AgroTM and in the control registered 100% of survival at 7 and 15 days after transplanting. However, a positive effect of VIUSID-AgroTM on the morphology and growth of in vitro plants of C. arabica L. cv. Red Caturra rojo was observed in relation the control. The highest heights of the plants were recorded in the treatments with VIUSID-AgroTM with no significant differences between both concentrations. At 30 days of cultivation in the ex vitro acclimatization phase there were significant differences between the treatments with VIUSID-AgroTM and the control only for plant height (Table 1).
There was no significant difference between the treatment with 0.5 mL L-1 of VIUSID-AgroTM and the control in dry weight, but both were significantly different to the treatment with 0.8 mL L-1. There were no differences between the treatments and the control in number of leaves, chlorophyll content, fresh weight, number of roots and the length of the main root at 30 days of cultivation. A good morphological development of the plants was observed in all the treatments (Figure 1).
At 60 days after transplanting in vitro plants developed in treatment with 0.5 mL L-1 of VIUSID-AgroTM registered the highest values in all variables. The VIUSID-AgroTM treatments developed plants with no significant between them, but both were significant different to the control.
The treatments with VIUSID-AgroTM were statistical similar in number of leaves, but only the treatment with 0.5 mL L-1 of VIUSID-AgroTM was significant superior to the control (Table 2). However, no significant differences were found in total chlorophyll content, number of roots and the length of the main root between VIUSID Agro™ and the control.
The treatment with 0.5 mL L-1 solution of VIUSID Agro™ obtained value of fresh weight and dry weight was statistically superior to the treatment with 0.8 mL L-1 solution of VIUSID Agro™ and the control. Plants developed in treatment control registered the lowest fresh weight and dry weight, which indicates that it did not have an efficient growth compared to the treatments where VIUSID-Agro™ was applied. The height difference among the treatments can be observed in Figure 2.
No information was found to date on the use of VIUSID Agro™ in the in vitro propagation of coffee. There is not any published research in the last phase of the process (ex vitro acclimatization), which is where high survival and growth of in vitro plants must be guaranteed so that they can be taken to the field.
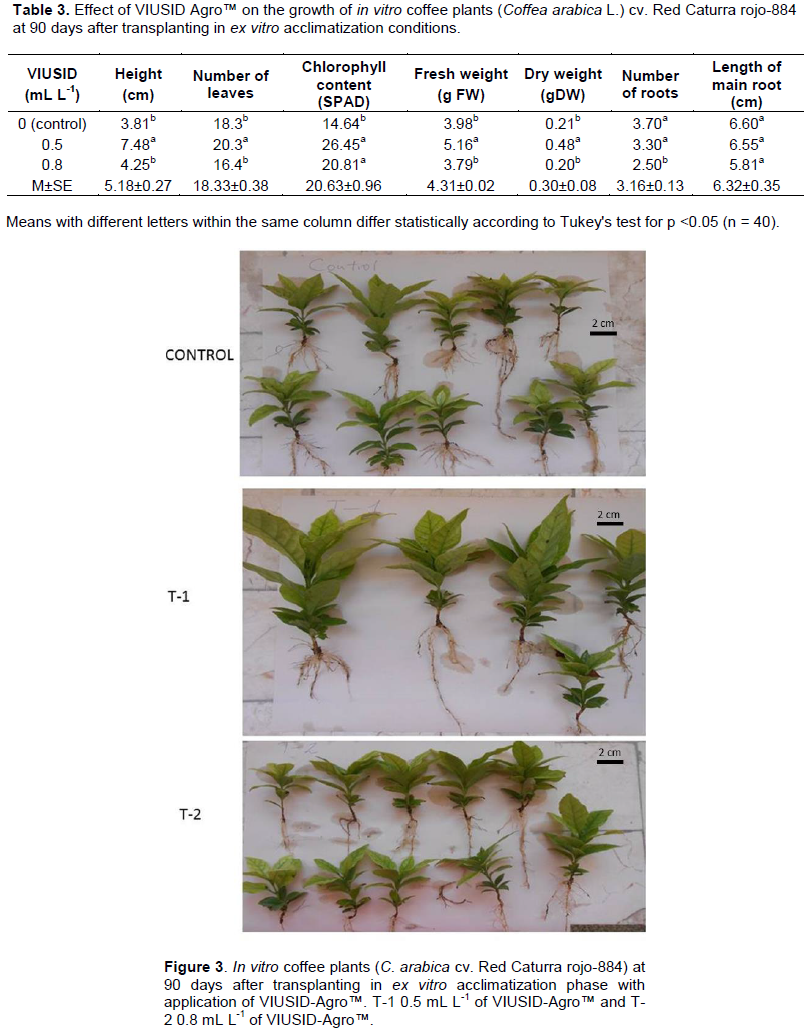
At 90 DAP, the treatment with 0.5 mL L-1 of VIUSID-Agro™ registered the highest values of plant height, number of leaves, fresh weight and dry weight were obtained with significant differences with the other treatments in the ex vitro acclimatization phase (Table 3). The height of the in vitro plants at 90 days after transplanting showed stimulatory effects on their growth by the biostimulant. This aspect is of utmost importance since it allows shortening their time period in the acclimatization phase, which is a crucial cost-saving component of the production process.
Another aspect to note that shows the positive effect of VIUSID-Agro™ is the SPAD absorbance values of chlorophyll reached at 90 days of cultivation. In the case of treatments with the application of this biostimulant, higher values of SPAD (total chlorophyll) were obtained in relation to the control (Table 3). Gómez-Kosky et al., (2020) reported similar results using in vitro plant of sugarcane during the ex vitro acclimatization phase with the same biostimulant.
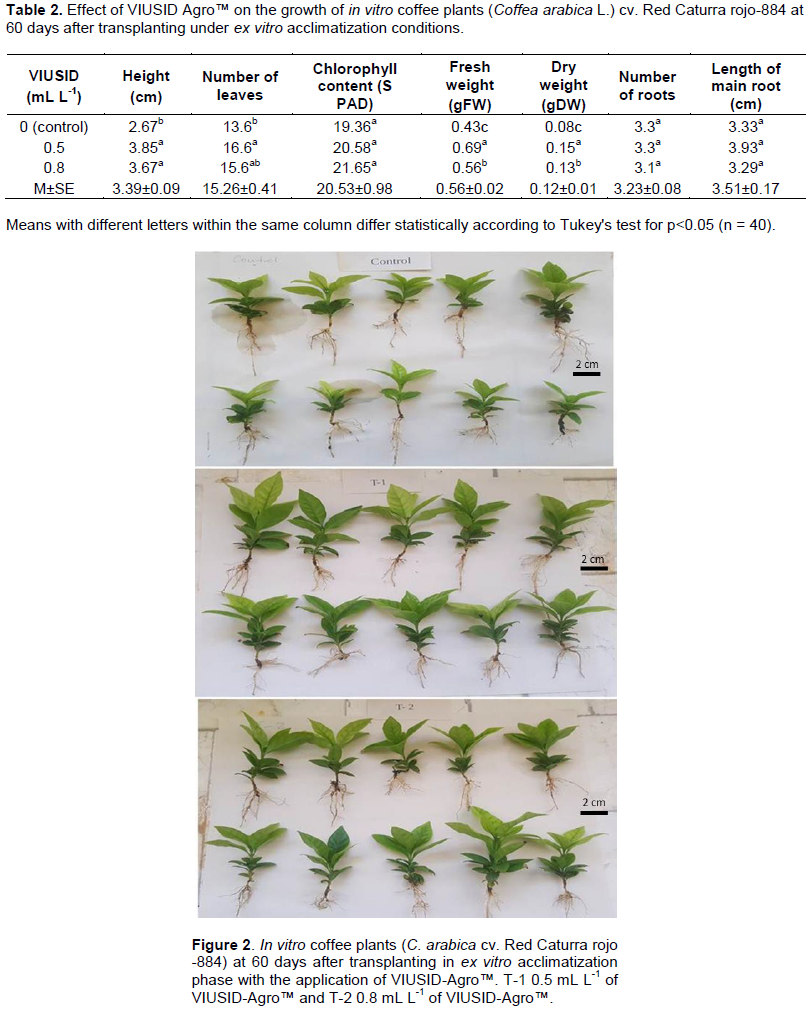
With regards to the number of roots, there were no significant differences between the control and 0.5 mL L-1 of VIUSID Agro™, but with the solution of 0.8 mL L-1 after 90 days. The two treatments and control were the same with respect to the length of the main root. The superiority of the results of 0.5 mL L-1 of VIUSID Agro™ could be observed over the other treatments with greater growth and development of plants (Figure 3). Accordingly, Pospisilova et al. (2007) noted that the fundamental aspect is that in vitro plants develop good rooting system because their nutrition depends largely on the functionality of the roots, as well as on nutrients accumulated in the seedlings before reaching ex vitro conditions.
Leaves play an important role in the development of in vitro plants. It was observed in this study that the biostimulant at 0.5 mL L-1 increased the number of leaves in the different treatments. In sugarcane (Saccharum spp cv. C90-469), Gómez-Kosky et al. (2020) reported that VIUSID Agro™ at 0.5 and 0.8 mL L-1 applied to in vitro plant during ex vitro acclimatization lead to leaf length and fresh weight exceeding those of the control.
Leaves produced in vitro are used for storage of carbon compounds, which are used in growth and development of the seedlings. When in vitro plants are transferred to ex vitro conditions, they maintain this function until new leaf emission. The first pair of leaves emitted in ex vitro conditions have autotrophic characteristics and temporarily accumulate photosynthate; where one part of these is retained by the leaf for growth and metabolism and the other part is exported outside the leaf to non-photosynthetic tissues and organs (Hazarika, 2003).
The results obtained in our study can be attributed to the composition of the VIUSID Agro™ formulation, which contains potassium phosphate that is necessary for the transfer and storage of energy in plants. In addition, it favors the formation of carbohydrates. The biostimulant also contains zinc sulfate that influences the creation and development of new tissues. Another component is glycine, which is a vital amino acid for growth, structural pillar of cytochromes and chlorophyll. This favors the formation of leaf tissue according to Catalysis (2019).
Besides, VIUSID Agro™ contains a group of amino acids, which are assimilated by plants through their leaves (Catalysis, 2019). The effects that can be produced when applying products with amino acids are of three types, one of which is hormonal: when amino acids enter plants, they stimulate the formation of chlorophyll, the auxin indole-3-acetic acid (IAA) and the production of vitamins and synthesis of numerous enzymatic systems (Simbaña and Carla, 2011). Another component of no less importance is folic acid that acts as a transporter of compounds. It is also a very important coenzyme in the metabolism of amino acids and in the synthesis of nitrogenous bases required for the formation of new tissues. Hence it positively influences growth of vegetative organs (Azcón-Bieto and Talón, 2008).
As reported by Catalysis (2019), the VIUSID Agro™ growth promoter was subjected to a biocatalytic process of molecular activation to improve its biological activity and the biochemical reactivity of all its molecules. It is an innovative liquid that, due to its components subjected to a biocatalytic process of molecular activation, make it different from the rest of the other products that can be similar to it on the market, since this process increases its effectiveness without altering its properties, producing a series of benefits when treating crops. The biocatalytic process of molecular activation greatly improves the biological activity and the biochemical reactivity of all antioxidant molecules. This activation method has been much more effective when applied to a much broader spectrum of water-soluble molecules.
Molecular activation is a process that increases the energy of molecules. This is possible due to the accumulation of additional electrons in the outer orbits of the molecules. Each component is activated separately following a specific protocol. This is what leads to its positive effects of growth and development in plants and hence an increase in yields.
VIUSID-Agro™ positively influenced ex vitro acclimatization of coffee plants. The greatest growth and development of plants from somatic embryos was achieved in the treatment with 0.5 mL L-1 of VIUSID-Agro™.
The authors have not declared any conflict of interests.
REFERENCES
|
Alarcón A, Barreiro P, Alarcón A, Díaz SY (2012). Efecto del Biobras 16 y el Fitomas E en algunos indicadores del crecimiento y el rendimiento del tomate (Solanum lycopersicum L.) variedad 'Vyta'. Revista Granma Ciencia 1:1-10.
|
|
|
|
Argoti CA, Belalcazar BN (2017). El mercado del café en los contextos mundial, nacional y regional. Revista UNIMAR 35(2):325-348.
|
|
|
|
|
Azcón-Bieto J, Talón M (2008). Fundamentos de Fisiología Vegetal. Segunda Edición. 638 p.
|
|
|
|
|
Catalysis (2019). VIUSID Agro, promotor del crecimiento.
|
|
|
|
|
Gómez-Kosky R, Núñez DJ, Reyes CE, Bernal AV, Bermúdez MC, Machado PA, Álvarez JF, Pineda ER, Kukurtcu B, Daniels DD (2020). Effect of VIUSID Agro and FitoMas-E on the ex vitro acclimatization of sugarcane plants (Saccharum spp.) cultivar C90-469. Sugar Tech 22:42-51.
Crossref
|
|
|
|
|
Hazarika B (2003). Acclimatization of tissue cultured plants. Current Science 85(12):1704-1712.
Crossref
|
|
|
|
|
MINAG (2018). Estadísticas oficiales del Ministerio de la Agricultura. La Habana, Cuba. Jan. 2019.
|
|
|
|
|
Montano R, Zuaznabar R, García A, Viñals M, Villar J (2007). FitoMas E, bionutriente derivado de la industria azucarera: Instituto Cubano de Investigaciones de los Derivados de la Caña de Azúcar (ICIDCA) sobre los derivados de la industria azucarera. La Habana 41(3):14-21.
|
|
|
|
|
Núñez M, Mazorra LM, Martínez M (2010). Los brasinoesteroides y las respuestas de las plantas a estrés abióticos. Una visión actualizada. Cultivos Tropicales 31(2):17-21.
|
|
|
|
|
Ortiz N, Barbón R, Capote A, Pérez A, Robaina M (2017). Caracterización morfológica en vivero de plantas de Coffea arabica L. cv Caturra rojo J-884 obtenidas por embriogénesis somática. Biotecnología Vegetal 17(4):251-257.
|
|
|
|
|
Peña CK, Rodr??guez FJC, Meléndrez JF (2015). Efecto de un promotor del crecimiento activado molecularmente sobre la germinación y la producción de frijol (Phaseolus vulgaris L.). Infociencia 19(3):1-12.
|
|
|
|
|
Piekielek W, Fox R, Toth JD, Macneal KE (1995). Use of a chlorophyll meter at the early dent stage of corn to evaluate nitrogen sufficiency. Agronomy Journal 87(3):403-408.
Crossref
|
|
|
|
|
Pospisilova J, Synkova H, Haisel D, Semoradova S (2007). Acclimatization of plantles to ex vitro conditions: effects of air, humidity, irradiance, CO2 concentration and abscisic acid. Acta Horticulturae 748:29-39.
Crossref
|
|
|
|
|
Simbaña C, Carla L (2011). Estudio de las propiedades físicas y funcionales de un hidrolizado enzimático de prote??na a escala piloto y su aplicación como fertilizante.
View. Accessed, May 2019.
|
|
|
|
|
Yakhin OI, Lubyanov AA, Yakhin IA, Brown PH (2017). Biostimulants in Plant Science: A Global Perspective. Frontiers in Plant Science 7(2049):1-32.
Crossref
|
|
|
|
|
Yu JQ, Feng LH, Hai WH, Hong YZ, Hua WM, Feng SY, Nogués S (2004). A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. Journal of Experimental Botany 55(399):1135-1143.
Crossref
|
|