Full Length Research Paper
ABSTRACT
The effects of arbuscular mycorrhizal fungi (AMF) and phosphorous (P) on the growth, nutrient uptake, chlorophyll content and some metabolites of eggplant (Solanum melongena L. VAR. Yalo) were determined under saline conditions, through a greenhouse experiment conducted for six weeks. Seedlings were planted in polythene bags previously filled with sand, 1 g of P and 50 g of AMF for all concentrations (0, 50, 100 and 200 mM NaCl) and supplied with a nutrient solution in a completely randomized design. The results of this study showed that increased concentrations of NaCl in the culture medium significantly (P<0.001) decreased the leaf area, stem height, dry biomass, chlorophyll content, K, Ca and Mg from 100 mM NaCl. The total soluble proteins, proline, total free amino acids, soluble carbohydrates, total phenolic and flavonoids contents increased significantly (P< 0.001) from 50 mM NaCl in all treatments. The findings indicate that P and AMF positively influenced all the study parameters compared to the treatment with NaCl only. The use of (P + AMF) alleviated the toxicity of NaCl, improved growth, physiological and biochemical parameters and its usage could be encouraged for better development of crops in salinity affected areas.
Key words: Solanum melongena, phosphorous, arbuscular mycorrhizal fungi, growth parameters, metabolites, salinity.
INTRODUCTION
Salt affected areas are daily on the rise and the main causes are natural and anthropogenic factors. In Arid and semi-arid zones, salts from basal rocks move to the upper layer of the soil through the process of water evaporation or floods (Kosová et al., 2013; Hand et al., 2021), as a natural cause and over irrigation as the anthropogenic cause. In the coastal regions, it is caused by invasion of land by sea water due to wind and rain (Munns and Tester, 2008). Infact, the main salt responsible of salinization is NaCl and in general, 20% of irrigated land and 2.1% of dry land agriculture are facing salt problem (Munns and Tester, 2008; Hand et al., 2021). The accumulation of salts at the rhizosphere have a negative impact on plant growth and metabolism. Nutrient availability and uptake is affected and soon alters plant function, productivity, photosynthesis, ion homeostasis and antioxidants phenomena. These changes definitively limit crop production and hence the eventual death of the plant (Kosová et al., 2013; Nouck et al., 2016; Hand et al., 2021). Increasing the tolerance of crop plants to salinity will assist plants resist the negative effects of salinity and also improve their productivity in saline soils.
Phosphorous plays a significant role in plant growth. It is involved in important metabolic processes, such as photosynthesis, respiration, nucleic acid synthesis, membrane synthesis and nitrogen synthesis (Gulmezoglu and Daghan, 2017). The presence of high amounts of soluble salts in the soil affects the availability of phosphorous and other minerals to plants (Khan et al., 2018). It could either be due to the formation of insoluble complexes with metals like Fe and Al, or its existence in the organic form (Shahriaripour et al., 2011). The application of phosphorous (P) to plants in saline soils improves crop growth and consequent production. Nevertheless, beyond a certain level of salinity, enzyme activity is reduced (Shahriaripour et al., 2011).
Arbuscular mycorrhizal fungi (AMF) are soil microorganisms that contribute to the improvement of growth in several plant species under saline conditions (Evelin et al., 2009; Chun et al., 2018). It forms extensive networks which improve soil structure and also produce biochemicals like glomalin that enhance the absorption of water and nutrients, from the soil to the host plant tissues (Yang et al., 2008; Mahdi et al., 2010; Scagel and Bryla, 2017). AMF absorbs phosphorous (P) and other minerals (Al-Fahdawi and Allawi, 2019) from the soil and make them available to the host plant. When plants are subjected to saline stress, AMF ensures that the plants take up sufficient water and nutrients by adjusting osmotic pressure and mineral imbalance in the soil respectively. AMF plays an important role in membrane stability and stimulates plants to produce their own defense, enhance photosynthetic pigments and maintain the osmotic and ionic balance of the cell (Yang et al., 2008) and it has a high ability to secrete the phosphatase enzyme, which converts organic phosphorus into mineral phosphorus (Al-Fahdawi and Allawi, 2019). Scagel and Bryla (2017) and Nouck et al. (2022) showed that AMF in the presence of salinity decreased the Na+ content and increased K, Ca, Mg, Na/K ratio and photosynthesis. Also, it has been reported that they modulate the biosynthesis of certain osmoprotectants such as proline, soluble carbohydrates, proteins and total free amino acids content improving and protecting the photosynthetic activity of plants (Yang et al., 2008; Scagel and Bryla, 2017).
The eggplant (Solanum melongena L.) is an economically important cash crop in the world with nutritional and medicinal properties and has great potentials as a food crop. Its global productions exceed 51.28 million tons/year (Kumar et al., 2020). It is a warm season crop, grown in various temperate and tropical parts of the world (Caruso et al., 2017) and cultivated primarily for its fruit. It provides significant nutritive and medicinal benefits due to the presence of vitamins, minerals, phenolics and antioxidants (Somawathi et al., 2015). It was hypothesized that arbuscular mycorrhiza and phosphorous influence the growth, nutrient uptake, chlorophyll content, and some metabolites of crop plants under saline stress. Therefore, the objective of this study was to evaluate the influence of arbuscular mycorrhizal fungi and phosphorous on the growth, nutrient uptake, chlorophyll content, biochemical constituents and non-enzymatic antioxidants of eggplant (Solanum melongena L. VAR. Yalo) under saline conditions.
MATERIALS AND METHODS
Study area and plant material
The research was carried out in a greenhouse located at New-Road Up-Station, Bamenda. The greenhouse was located at latitude 5° 56’ North and longitude 10° 15’ East, in Mezam division of the North West region of Cameroon. This area is located at 1614 m above sea level. The work was carried out from December 2019 to May 2020, average rain fall and temperatures are 865 mm/year and 30°C and relative humidity is close to 86%. Prevailing winds carry the tropical monsoon. The seeds of Solanum melongena Var. yalo used for the work and arbuscular mycorrhiza fungi (Bio 1: Gigaspora margarita + Acaulospora tuberculata and Bio 2: Scutellospora gregaria) were obtained from the Institute of Agronomic Research and Development (IRAD) Nkolbisson, Yaounde-Cameroon.
Plant growth conditions and salt treatments
Eggplant seeds were sterilized after a viability test with 3% of sodium hypochlorite for 10 min, washed ten times with demineralized water and transplanted into 3 L polythene bags previously filled with 3 kg of sterilized sand, with one plant each and five replications per treatment. The plants were arranged in a complete randomized block design and daily enriched with a modified nutrient solution (in g/L): Of 150 g Ca(NO3)2, 70 g KNO3, 15 g Fe-EDTA, 0.14 g KH2PO4, 1.60 g K2SO4, 11 g MgSO4, 2.5 g CaSO4, 1.18 g MnSO4, 0.16 g ZnSO4, 3.10 g H3BO4, 0.17 g CuSO4 and 0.08 g MOO3 (Hoagland and Arnon, 1950). The pH of the nutrient solution was adjusted to 7.0 by adding HNO3 0.1 mM. Plants were subjected to different salt concentrations (0, 50, 100 and 200 mM NaCl) with 0 mM NaCl as a control in the culture medium for a period of six weeks to determine the physiological and biochemical responses of cultivars to salt stress. The average day and night temperatures in the greenhouse were between 25 and 18°C, respectively during the growth period with average relative air humidity of 72%. Parameters were evaluated under greenhouse conditions: Stem height, leaf area, dry biomass of roots and shoots, chlorophyll (a+b), proline, soluble carbohydrates, total phenol and flavonoids content and mineral (Na, K, Ca and Mg contents) of roots and shoots.
Growth parameters
The leaf area, stem height, dry weights were recorded after six weeks. The leaf area was calculated using the formula, surface area (cm2) = 1/3 (length × width) (Nouck et al., 2016). The eggplant parts were dried separately (roots and shoots) at 65°C for 72 h in an oven and their dry biomasses were determined (Nouck et al., 2016). The stem height was determined with a ruler.
Mineral distribution
The Pauwels et al. (1992) method was used to determined potassium, calcium, sodium and magnesium in roots and shoots. 2 g of dried organs were separately reduced to ashes by heating at 550°C for 4 h and thoroughly mixed with 250 mL of deionized water. The filtrate was analysed with an atomic absorption spectrophotometer (Rayleigh WFX-100).
Chlorophyll content
The Arnon (1949) method was used to determine chlorophyll (a+b) content. 0.80 g sample of fresh leaves were crushed and their contents extracted with 80% of alkaline acetone (v/v). The filtrate was analyzed using a spectrophotometer (Pharmaspec model UV-1700) at 645 and 663 nm wavelengths.
Metabolites
For the analysis of metabolites, all samples were obtained from the leaves.
Total soluble protein content
The total soluble protein content was determined using the Bradford (1976) method. An appropriate volume (from 0 - 100 µl) of crude extract was put into a test tube and the total volume was augmented to 100 µl with distilled water. 1 ml of Bradford solution was added to the sample. Then the mixture was thoroughly mixed with a vortex mixer. The absorbance was read at 595 nm with a spectrophotometer UV (PG instruments T60) after 2 min. The standard curve obtained was used to determine PR content.
Proline content
The proline content was estimated using Bates et al. (1973) method. 0.5 g of fresh leaves were weighed, crushed and put inside a flask. 10 mL of 3% aqueous sulphosalicylic acid was poured in the same flask. The mixture was homogenized, and then filtered with a Whatman No1 filter paper. 2 mL of filtered solution was poured into a test tube, and then 2 mL of glacial acetic acid and ninhydrin acid were respectively added into the same tube. The test tube was heated in a warm water bath for 1 h. The reaction was stopped by placing the test tube in an ice bath. 4 mL of toluene was added to the test tube and stirred. A purple-coloured mixture was obtained and its absorbance was read at 520 nm by spectrophotometer UV (Pharmaspec model UV-1700). The proline concentration was determined using the standard curve (µg/g FW).
Soluble carbohydrate content
The soluble carbohydrate content was obtained using phenol-sulphuric acid (Dubois et al., 1956). The fresh leaves (1 g) were ground in 5 mL of 80% ethanol and filtered with the Whatman No 1 filter paper. The extract was diluted with deionized water to make up 50 mL. 1 mL of sample was poured in test tube, followed by the addition of 1 mL of phenol solution and 5 mL of sulphuric acid. The mixture was then swirled. The absorbance was read at 490 nm using a spectrophotometer (Pharmaspec UV-1700 model). The quantity of CH was deduced from the glucose standard curve.
Total free amino acids content
The total free amino acids content was determined by the ninhydrin method (Yemm and Cocking, 1955). Fresh leaves (1 g) were ground in 5 mL of ethanol 80%, amino acids were then extracted using reflux technique in boiling ethanol for 30 min. After decanting, the supernatant was filtered using Whatman N°1 filter paper. The filtrate was collected and the residue used to repeat the extraction. The two filtrates were mixed and the raw extract of amino acid content was measured using ninhydrine method. The absorbance of purplish-blue complex was read at 570 nm wavelength. The standard curve was established using 0.1 mg/mL of glycine.
Flavonoids content
The flavonoids content of crude extract was determined by using the aluminum chloride colorimetric method (Chang et al., 2002). 1 mg/mL of the extract was prepared by dissolving 1 mg of eggplant leaves in 1 mL of ethanol. 50 µL of crude extract (1 mg/mL ethanol) was pipetted and 950 µL of methanol added to made it up to 1 mL with methanol, mixed with 4 mL of distilled water and then 0.3 mL of 5% NaNO2 solution; 0.3 mL of 10% AlCl3 solution was added after 5 min of incubation, and the mixture was allowed to stand for 6 min. Then, 2 mL of 1 mol/L NaOH solution was added, and the final volume of the mixture was brought to 10 mL with double-distilled water. The mixture was allowed to stand for 15 min, and absorbance was recorded on spectrophotometer (Pharmaspec UV-1700 model) at 510 nm. FLA content was calculated from a grutin calibration curve, and the result was expressed as grutin equivalent per gram dry weight.
Total phenolic content
The total phenolic content of the extract was determined by the Folin Ciocalteu method (Marigo, 1973). 1 g of fresh leaves were ground at 4?C in 3 mL of 0.1 N HCl. After incubation at 4?C for 20 min, the homogenate was centrifuged at 6000 g for 40 min. The supernatant was collected, the pellet re-suspended in 3 mL of 0.1 N HCl and centrifuged as previously. The two supernatants were mixed and 15 µL of the mixture was mixed with 100 µL Folin-Ciocalteu reagents and 0.5 mL of 20% Na2CO3 added before incubating at 40?C for 20 min. After this time, the absorbance was read at 720 nm using a spectrophotometer (Pharmaspec UV-1700 model). A standard curve was established using chlorogenic acid. TP content was expressed as mg/g fresh weight.
Statistical analysis
The experiment was performed using a completely randomizeddesign. All data were presented in terms of mean (± standard deviation), statistically analysed using Graph pad Prism version 5.01. The normality of data distribution was checked using the Shapiro-Wilk test (Shapiro and Wilk, 1965): The statistical difference between the groups and between the treatments was performed using the analysis of variance (ANOVA) and where the F- values were found to be significant, the treatment means were separated by Least Significant Difference (LSD) at 5% probability level using Duncan’s Multiple Range Test (DMRT).
RESULTS
Plant growth
The growth parameters were generally affected with intake doses of NaCl in the culture medium (Figure 1). The treatment (NaCl + P) significantly (P<0.05) improved in all concentrations compared to the treatment with NaCl only. The treatment (NaCl + P+ B2) significantly (P<0.01) improved in all concentrations compared to the treatment with NaCl only and (NaCl + P). The treatment (NaCl + P+ B1) significantly (P<0.01) improved in all concentrations and above all treatments except the treatment (NaCl + P+ B (1+2)) which significantly (P<0.001) improved in all concentrations and treatments.
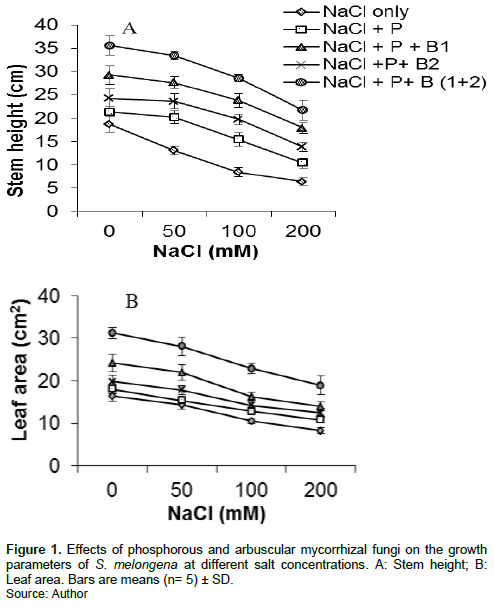
Dry weight partitioning
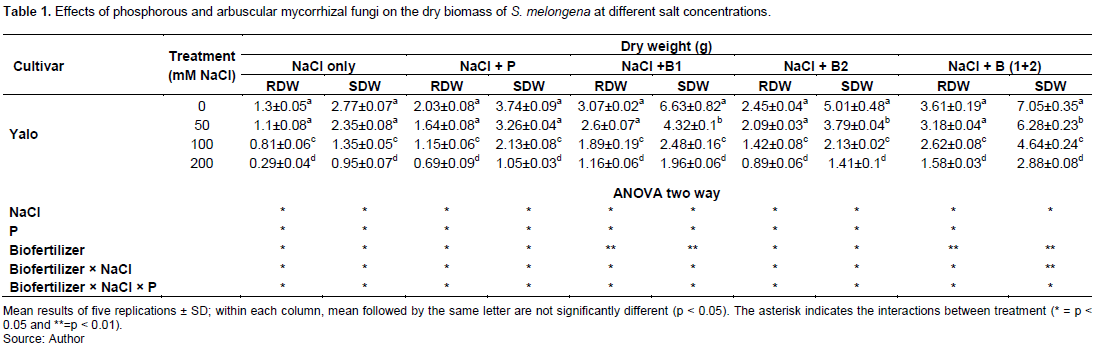
The dry weight of plant organs decreased significantly (P<0.05) in the culture medium with intake doses of NaCl for all treatments (Table 1) from 100 mM NaCl. The treatment (NaCl + P+ B (1+2)) significantly (P<0.001) improved in all concentrations compared to the treatment with NaCl only [(NaCl + P), (NaCl + P+ B2) and (NaCl + P + B1)]. For all treatments with NaCl + P+ AMF, a general improvement was observed in the dry biomass at all concentrations compared to treatments lacking AMF (NaCl and (NaCl + P). The interactions between AMF × NaCl and between AMF × NaCl × P were significant (P<0.05) in all treatments and concentrations except in shoots dry weight of treatment [(NaCl + P+ B (1+2)].
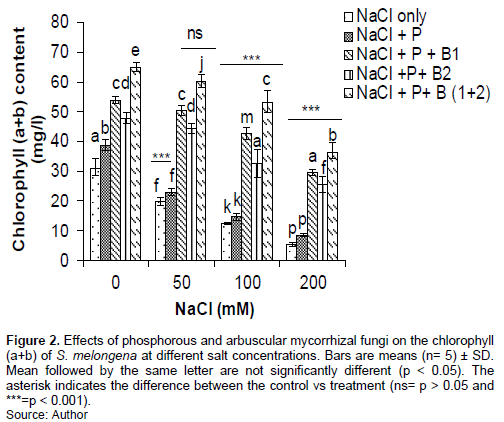
Chlorophyll (a+b) content
The chlorophyll (a+b) content in the leaves of eggplant decreased significantly (P <0.001) from 50 mM NaCl for treatments with NaCl only and NaCl + P (Figure 2). The chlorophyll (a+b) decreased significantly (P <0.001) from 100 mM NaCl for treatments with NaCl + P+ B2, NaCl + P + B1 and NaCl + P+ B (1+2). The treatment (NaCl + P+ B (1+2)) significantly (P<0.001) improved in all concentrations compared to the other treatments (Figure 2).
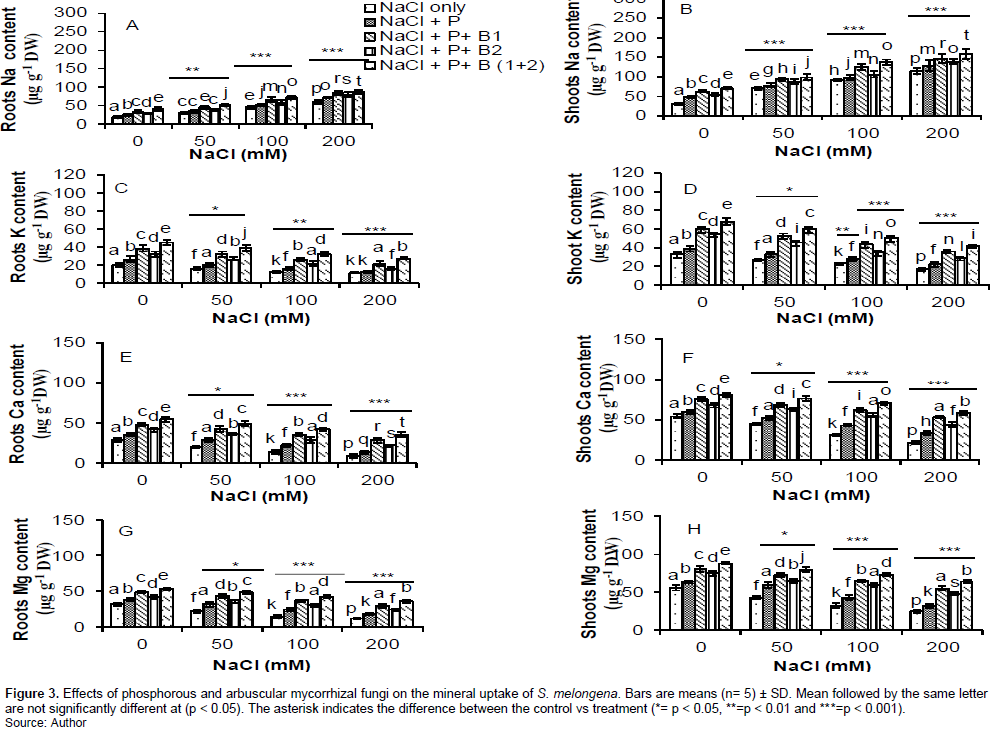
Mineral distribution
The results of mineral uptake showed that Na+ increased significantly (P<0.001) with intakes doses of NaCl in the culture medium in plant partitions while others minerals (K+, Ca2+ and Mg2+) decreased significantly (P<0.001) at the same concentrations in all treatments (Figure 3). The treatment (NaCl + P+ B (1+2)) significantly (P<0.001), (P<0.01) and (P<0.05) improved in all concentrations compared to the treatment with NaCl only and NaCl + P, NaCl + P+ B2 and NaCl + P + B1 respectively in the culture medium (Figure 2).
Metabolites
Primary metabolites
The proline content (PRO), soluble carbohydrates (CH), total amino acids (FAA) and total soluble proteins (PR) of eggplant leaves increased significantly (P<0.001) with increased concentrations of NaCl in the culture medium for all treatments (Figure 4). The biochemical constituents of S. melongena increased significantly with increased NaCl concentrations. The PRO content results showed that the treatment (NaCl + P+ B (1+2)) significantly (P<0.001, P<0.01 and P<0.05) improved in all concentrations compared to the treatments with NaCl only and NaCl + P, NaCl + P+ B2 and NaCl + P + B1, respectively (Figure 4). The same pattern was observed with CH, FAA and PR at the same concentrations in the culture medium. In this study all treatments with AMF showed higher values than those without AMF (Figure 4).
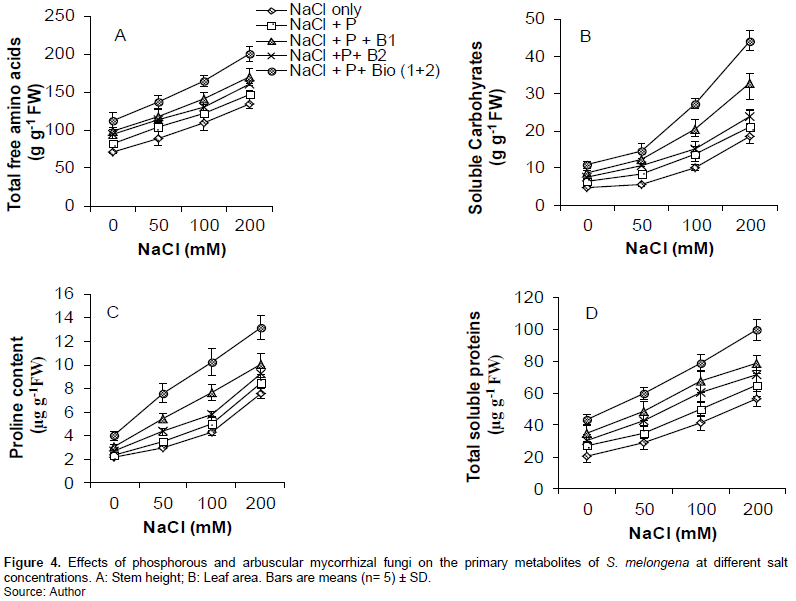
Secondary metabolites
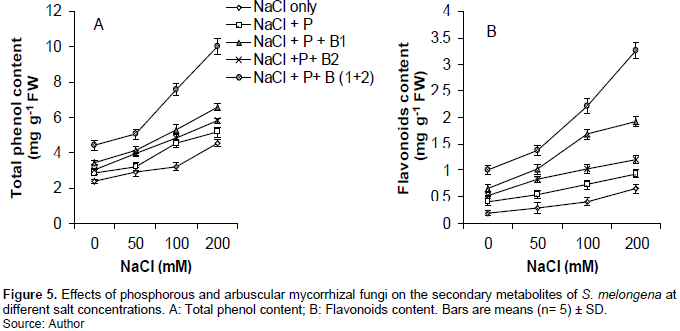
Total phenol content experienced an upward trend significantly (P<0.001) with increased salinity in all treatment. The treatment (NaCl + P+ B (1+2)) significantly (P<0.001, P<0.01 and P<0.05) improved in all concentrations compared to the other treatments in general. The results obtained for flavonoids content showed a similar pattern like those of total phenol content in the same treatments and concentrations (Figure 5).
DISCUSSION
The growth parameters generally decreased with intake doses of NaCl in the culture medium. These results corroborate those of Heidari et al. (2014). According to the study, the loss of water by plant cells caused the loss of cell turgor and shrinkage, reducing the rate of cell elongation. This contributed to the formation of shorter plant stems. In the same line, Kamran et al. (2020) in conformity with this trend reported that photosynthetic rates were retarded under high salinity due to decreased efficiency of growth hormones, resulting in decreased stem height. Heidari et al. (2014) and Kamran et al. (2020) justify the decrease in leaf area by stating that the loss of water by plant cells caused the loss of cell turgor and shrinkage, reducing the rate of cell elongation. Similar results were reported by Shahriaripour et al. (2011) on Pistachio seedlings. Even though their findings indicated that plant response to phosphorous addition under saline conditions greatly depended on the species and cultivar examined. Tang et al. (2019) also reported that stem height increased at low saline concentrations because of increased phosphorous mobility. Munns and James (2003); Shahriaripour et al. (2011) and Tang et al. (2019) revealed that P alleviates the negative impact of salinity on plant growth, particularly the leaf area of certain plants compared to those treated with NaCl only. For plants exposed to AMF in general, Evelin et al. (2009) showed that AMF alleviates the negative effects of salt stress by enhancing nutrient uptake, photosynthetic activities and water absorption; consequently contributing to the improvement of the growth of several plant species under saline conditions.
The dry weight of plant organs decreased in the culture medium with intake doses of NaCl for all treatments. Previous authors like Nouck et al. (2016) on Lycopersicum esculentum L. and Hand et al. (2017) on Capsicum annuum L. reported that plant dry biomass was subject to photosynthetic activity and mineral distributions. Inhibiting sufficient uptake of minerals to the tissues and a decrease in the photosynthetic efficiency of the plant led to a corresponding decrease in dry biomass. Additionally, Heidari (2012) explained that the reduced ability of plants to take in water under saline conditions caused slower growth. Also, excessive salts from the transpiration stream injured the cells of transpiring leaves. This is in line with our results for the treatment with NaCl only. The addition of phosphorus reflected an improvement in the results for all treatments and concentrations as compared with those of NaCl only. According to Tang et al. (2019), low Na+ concentrations stimulate Na+-dependent high affinity phosphate uptake which are involved in the synthesis of sucrose and starch during photosynthesis. Hence, an improvement in plant dry weight through the accumulation of dry matter. The interactions between AMF + NaCl have a positive impact on plant biomass though the extent usually varies with the plant type and soil saline concentration. For all treatments with NaCl + P+ AMF a general improvement was observed in the dry biomass at all concentrations compared to treatments lacking AMF (NaCl and (NaCl + P). This could be due to the fact that AMF and P contribute to alleviate the detrimental effects of NaCl by enhancing the water uptake, mineral nutrition and photosynthetic activities of the plants. These changes were directly responsible for increased biomass (Chun et al., 2018). The interactions between AMF + NaCl + P tend to mitigate the adverse effects of NaCl on plant biomass, depending on the crop plant and saline concentration.
The chlorophyll (a+b) content in the leaves of eggplant decreased from 50 mM NaCl for the treatments NaCl only and NaCl + P. This result is in accordance with those of Murkute et al. (2006); Kaya et al. (2009) and Abdel Latef and Chaoxing (2011). They explained that the reduction in water potential, the antagonistic effects of NaCl on N absorption, the decreased uptake of certain nutrients like Mg needed for chlorophyll biosynthesis and the suppression of activity of specific enzymes required for the synthesis of photosynthetic pigments contribute to the decrease in chlorophyll. Yang et al. (2010) further reported that phosphorous application increased total chlorophyll content of plants by assisting in the production of photosynthetic pigments which are responsible for photosynthesis and also increase the stability of chlorophyll. This explains why treatment with NaCl + P improved compared to the treatment with NaCl only.
Several researchers such as Abdel Latef and Chaoxing (2011) and Nouck et al. (2022) indicated that AMF alleviate the negative effects of NaCl on chlorophyll content resulting in a higher chlorophyll concentration in plant leaf under saline conditions. The fungi are able to alleviate the antagonistic effects of Na+ on Mg2+ uptake under salt stress (Giri et al., 2003). Our results agree with the findings of Cekic et al. (2012). He reported that AMF and P were responsible for the high chlorophyll contents. AMF and P caused more uptake of Mg2+; boosting the synthesis of chlorophyll, regulating the enzymes in chlorophyll synthesis while reducing chlorophyllase, maintaining cell membrane integrity and stability of the chlorophyll pigments and nutritional improvements. The significant improvement of chlorophyll in the treatment with NaCl + P + B (1+2) obtained can be justified by the type of biofertilizers used and the crop plant.
The Na+ increased with intakes doses of NaCl in the culture medium in plant partitions while other minerals (K+, Ca2+ and Mg2+) decreased at the same concentrations in all treatments. According to Nouck et al. (2016) and Hand et al. (2017), the competition of Na+ and K+ for aerial plants resulted in greater accumulation of Na+ in the shoots than in the roots. Ca2+ ameliorates salt stress through osmotic adjustments by enhancing ion uptake. In saline medium, Na+ replace Ca2+, resulting in the disintegration of cell membranes and cell walls. Although Na+ increased with increased salinity in both shoots and roots while K+ decreased, with the addition of phosphorous an improvement was recorded in the mineral uptake. The results of NaCl + P are in agreement with the works of Bargaz et al. (2016) and Gulmezoglu and Daghan (2017). Their findings suggested that the uptake of Na+ is related to the concentration gradient, even in the presence of phosphorous. According to Bargaz et al. (2016), K+, Ca2+ and Mg2+ work together with phosphorous to minimize the adverse effects of Na+ on plants and the effect was expressed in the improved growth parameters of the plants.
The treatment (NaCl + P+ B (1+2)) improved in all concentrations compared to the treatment with NaCl only and NaCl + P, NaCl + P+ B2 and NaCl + P + B1 respectively in the culture medium. According to Giri et al. (2007) and Abdel Latef and Chaoxing (2011), the Na+ concentration is lower in mycorrhizal than non mycorrhizal plants under salinity and could be explained by dilution effects of plant growth enhancement caused by AMF colonization. In this study, the negative effects of NaCl were attenuated by the association of phosphates and both AMF, enhancing the absorption of K+, Ca2+ and Mg2+. This led to the reinstatement of the ionic balance (Beltrano et al., 2013; Alqarawi et al., 2014) since K+ and Ca2+ are involved in energy metabolism and Mg2+ is a central component of chlorophyll pigments. In addition the improvement in the uptake of these minerals also depends on the treatment, the type of plant, AMF and the mixture of AMF.
The primary metabolites increased with NaCl in the culture medium. It has been commonly reported by Kosovà et al. (2013), Nouck et al. (2016) and Hand et al. (2017) that biochemical constituents are salt-tolerance indicators and possess osmoprotective qualities. They showed that PR increased due to regulatory adjustments to stress, resulting in its active synthesis. This is because PR enhances plant salt tolerance. Its production is considered as an adaptive mechanism of plants to salinity. The findings of Nouck et al. (2016) indicated that the increase in FAA with increased salinity was due to the reduction of osmotic potential to maintain the turgid potential. According to Abdallah et al. (2016), osmotic stress caused by physiological drought is responsible for decreased osmotic potential, leading to the active synthesis and accumulation of CH. These results are consistent with those of Bargaz et al. (2016) and Gulmezoglu and Daghan (2017). They reported that soluble carbohydrates, total soluble proteins, total free amino acids and proline are produced in large amounts in response to salinity stress as an adaptive mechanism. This is because they function as osmoprotectants. The treatment (NaCl + P) significantly (P<0.05) improved in all concentrations compared to the treatment with NaCl only. In this study, application of P was instrumental in the increase in photosynthetic pigments, which contributed to the significant increase in CH, PR, FAA and PRO in salinity stress.
This result indicates that the effect of AMF with P application constitutes one of the main reasons for increasing plant tolerance to salinity. According to Beltrano et al. (2013), salt-stress induces P deficiency in plants by reducing P uptake or translocation. This suggests that AMF enhances the plant growth by mechanisms that may not be related to improvement of P nutrition. Additionally, Al-Karaki (2000) and Giri et al. (2007) suggests that alleviation of salt stress by AMF includes improvement in P nutrition. They showed that improvement in P plant status is an important mechanism of salinity stress tolerance in mycorrhizal plants. In this work, all treatments containing AMF + P had elevated increase in primary metabolites as compared to the increase observed in plants treated with NaCl only. It is evident that the combination of P and AMF augment the production of primary metabolites in plants subjected to salinity stress. The quantity of primary metabolites produced is subject to crop type, type of biofertilizer and the mixture of biofertilizers.
Total phenol content and flavonoids experienced an upward trend with increased salinity in all treatments. Taïbi et al. (2016) maintain that the accumulation of total phenols and flavonoids are physiological responses to plant stress. They reduced oxidative damage by scavenging ROS, while maintaining chlorophyll levels and cell turgor to protect photosynthetic activities (Meguekam et al., 2014).
The quantity of TP and FLA in eggplants increased with the supply of P to the culture medium. In conformity with this result, Pontigo et al. (2018) explained that P improves plant efficiency to accumulate non-enzymatic antioxidants under stress conditions. It encourages the production of more non-enzymatic antioxidants so as to counter the effects of stress on the plants.
More flavonoids and total phenols were produced in eggplants that received P and AMF. In conformity with this result, Hashem et al. (2018) reported that AMF enhanced the accumulation of non-enzymatic antioxidants which assisted to counter the effects of stress on the plants. In our study, the mixture of P + Bio1 and Bio2 significantly improved non-enzymatic antioxidants compared to others treated with AMF+P. This could be due to the combined effect of 3 AMFs (Bio 1: Gigaspora margarita + Acaulospora tuberculata and Bio 2: Scutellospora gregaria).
CONCLUSION
From this study it can be concluded that Arbuscular mycorrhizal fungi associate with phosphorous to alleviate detrimental effects of salinity on the growth, nutrient uptake (higher Na+ and lower K+, Ca2+ and Mg2+ concentrations in leaf tissue), chlorophyll content and some metabolites of eggplant under saline conditions. The stem height, the leaf area, the dry biomass, the mineral uptake, the Chlorophyll (a+b) decreased with salinity doses of NaCl from 100 mM NaCl for the treatment with NaCl + P + AMF and from 50 mM for the treatment with NaCl only while osmolytes increased from 50 mM NaCl. The AMF + P enhanced the accumulation of all the study parameters than non-AMF + P plants under salinity. The addition of P, Bio 1(Gigaspora margarita + Acaulospora tuberculata) and Bio 2 (Scutellospora gregaria) in the culture medium caused significant increase in all the studied parameters and treatments. Phosphorous restrains the negative effects of salinity and associate with biofertilizers to mitigate the impact of salinity on the eggplants, resulting in significant improvements. The high accumulation of metabolites with salinity doses could be added as indicators of early identification and osmotic adjustment ability for salt-tolerant plants in salt stress conditions. Eggplants could be cultivated in soils with moderate salinity. Using AMF and P as an alternative way of decreasing NaCl stress in plants will be more beneficial as it maintains soil fertility.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Abdallah MMS, Abdelgawad ZA, EL-Bassioumy HMS (2016). Alleviation of the adverse effects of salinity stress using trehalose in two rice varieties. South African Journal of Botany 103:275-282. |
|
|
Abdel Latef AA, Chaoxing H (2011). Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Scientia Horticulturae 127(3):228-233. |
|
|
Al-Fahdawi AJ, Allawi MM (2019). Impact of biofertilizers and nano potassium on growth and yield of eggplant (Solanum melongena L.). Plant Archives 19(2):1809-1815. |
|
|
Al-Karaki G (2000). Growth of mycorrhizal tomato and mineral acquisition under salt stress. Mycorrhiza 10(2):51-54. |
|
|
Alqarawi AA, Abd_Allah EF, Hashem A (2014). Alleviation of salt-induced adverse impact via mycorrhizal fungi in Ephedra aphylla Forssk. Journal of Plant Interactions 9(1):802-810. |
|
|
Arnon DI (1949). Copper enzymes in isolated chloroplasts. Polyphenylodase in Beta vulgaris. Plant Physiology 24(1):1-15. |
|
|
Bargaz A, Nassar RMA, Rady MM, Gaballah MS, Thompson SM, Brestic M, Schmidhalter U, Abdelhamid MT (2016). Improved Salinity Tolerance by Phosphorus Fertilizer in Two Phaseolus vulgaris Recombinant Inbred Lines Contrasting in Their P-Efficiency. Journal of Agronomy and Crop Science 202(6):497-507. |
|
|
Bates L, Waldren RP, Teare ID (1973). Rapid determination of free proline for water stress studies. Plant and Soil 39(1):205-207. |
|
|
Beltrano J, Ruscitti M, Arango MC, Ronco M (2013). Effects of arbuscular mycorrhizal inoculation on plant growth, biological and physiological parameters and mineral nutrition in pepper grown under different salinity and p levels. Journal of Soil Science and Plant Nutrition 13(1):123-141. |
|
|
Bradford MM (1976). A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Proteins Utilizing the Principle of Protein-Dye Binding. Analytical Biochemistry 72(1-2):248-254. |
|
|
Caruso G, Pokluda R, Sekara A, Kalisz A, Jezdinsky A, Kopta T, Grabowska A (2017). Agricultural practices, biology and quality of eggplant cultivated in central Europe. A review. Horticultural Science 44(4):201-212. |
|
|
Cekic FO, Unyayar S, Ortas I (2012). Effects of arbucular mycorrhizal inoculation on biochemical parameters in Capsicum annuum grown under long term salt stress. Turkish Journal of Botany 36(1):63-72. |
|
|
Chang CC, Yang MH, Wen HM, Chern JC (2002). Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. Journal of Food and Drug Analysis 10(3):178-182. |
|
|
Chun SC, Paramasivan M, Chandrasekaran M (2018). Proline Accumulation Influenced by Osmotic Stress in Arbuscular Mycorrhizal Symbiotic Plants. Frontiers in Microbiology 9:2525. |
|
|
Dubois M, Gilles KA, Hamil JK, Rebers PA, Smith F (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry 28(3):350-356. |
|
|
Evelin H, Kapoor R, Giri B (2009). Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Annals of Botany 104(7):1263-1280. |
|
|
Giri B, Kapoor R, Mukerji KG (2003). Influence of arbuscular mycorrhizal fungi and salinity on growth, biomass and mineral nutrition of Acacia auriculiformis. Biology and Fertility of Soils 38(3):170-175. |
|
|
Giri B, Kapoor R, Mukerji KG (2007). Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues Microbial Ecology 54(4):753-760. |
|
|
Gulmezoglu N, Dagha H (2017). The interactive effects of phosphorous and salt on growth, water potential and phosphorous uptake in green beans. Applied Ecology and Environmental Research 15(3):1831-1842. |
|
|
Hand MJ, Taffouo VD, Nouck AE, Nyemene KPJ, Tonfack LB, Meguekam TL, Youmbi E (2017). Effects of Salt Stress on Plant Growth, Nutrient Partitioning, Chlorophyll Content, Leaf Relative Water Content, Accumulation of Osmolytes and Antioxidant Compounds in Pepper (Capsicum annuum L.) Cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 45(2):481-490. |
|
|
Hand MJ, Nono GV, Tonfack LB, Taffouo VD, Youmbi E (2021). Nutrient Composition, Antioxidant Components and Ascorbic Acid Content Response of Pepper Fruit (Capsicum annuum L.) Cultivars Grown under Salt Stress. International Journal of Biology 2(1):43-70. |
|
|
Hashem A, Alqarawi AA, Radhakrishnan R, Al-Arjani AF, Aldehaish HA, Egamberdieva D, Allah EFA (2018). Arbuscular mycorrhizal fungi regulate the oxidative system, hormones and ionic equilibrium to trigger salt stress tolerance in Cucumis sativus L. Saudi Journal of Biological Sciences 25(6):1102-1114. |
|
|
Heidari A, Bandehagh A, Toorchi M (2014). Effects of NaCl Stress on Chlorophyll Content and Chlorophyll Fluorescence in Sunflower (Helianthus annus L.) Lines. Yuzuncu Y?l University Journal of Agricultural Sciences 24(2):111-120. |
|
|
Heidari M (2012). Effects of salinity stress on growth, chlorophyll content and osmotic components of two basil (Ocimum basilicum L.) genotypes. African Journal of Biotechnology 11(2):379-384. |
|
|
Hoagland DR, Arnon DI (1950). The water-culture method for growing plants without soil. University of California, College of Agriculture, Berkley; 1-34. |
|
|
Kamran M, Parveen A, Ahmar S, Malik Z, Hussain S, Chattha MS, Saleem MH, Adil M, Heidari P, Chen J (2020). An Overview of Hazardous Impacts of Soil Salinity in Crops, Tolerance Mechanisms and Amelioration through Selenium and Supplementation. International Journal of Molecular Sciences 21(1):148. |
|
|
Kaya C, Ashraf M, Sonmez O, Aydemir S, Tuna AL, Cullu MA (2009). The influence of arbuscular mycorrhizal colonisation on key growth parameters and fruit yield of pepper plants grown at high salinity. Scientia horticulturae 121(1):1-6. |
|
|
Khan Z, Islam A, Azom G, Amin S (2018). Short Term Influence of Salinity on Uptake of Phosphorous by Ipomoea aquatic. International Journal of Plant and Soil Science 25(2):1-9. |
|
|
Kosová K, Prášil TI, Vítámvás P (2013). Protein Contribution to Plant Salinity Response and Tolerance Acquisition. International Journal of Molecular Sciences 14(4):6757-6789. |
|
|
Kumar A, Sharma V, Jain BT, Kaushik P (2020). Heterosis Breeeding in Eggplant (Solanum melongena L.): Gains and Provocations. Plants 9(3):403. |
|
|
Mahdi SS, Hassan GI, Samoon SA, Rather HA, Dar SA, Zehra B (2010). Bio-fertilizers in organic agriculture. Journal of Phytology 2(10):42-54. |
|
|
Marigo G (1973). On a fractionation method and estimation of the phenolic compounds in plant. Analysis 2(2):106-110. |
|
|
Meguekam TL, Taffouo VD, Grigore MN, Zamfirache MM, Youmbi E, Amougou A (2014). Differential responses of growth, chlorophyll content, lipid perodxidation and accumulation of compatible solutes to salt stress in peanut (Arachis hypogaea L.) cultivars. African Journal of Biotechnology 13(50):4577-4587. |
|
|
Munns R, Tester M (2008). Mechanisms of Salinity Tolerance. Annual Review of Plant Biology 59:651-681. |
|
|
Munns R, James RA (2003). Screening methods for salinity tolerance: a case study with tetraploid wheat. Plant and Soil 253(1):201-218. |
|
|
Murkute AA, Sharma S, Singh SK (2006). Studies on salt stress tolerance of citrus rootstock genotypes with arbuscular mycorrhizal fungi. Horticultural Science 33:70-76. |
|
|
Nouck AE, Taffouo VD, Tsoata E, Dibong SD, Nguemezi ST, Gouado I, Youmbi E (2016). Growth, Biochemical Constituents, Micronutrient Uptake and Yield Response of Six Tomato (Lycopersicum esculentum L.). Journal of Agronomy 15(2):58-67. |
|
|
Nouck AE, Ekwel SS, Momnjoh EK, Shang EW, Taffouo VD (2022). Effects of Mycorrhizal Biofertilizer on the Biochemical Constituent, Nutrient Uptake, Chlorophyll Content, Growth and Agronomic Parameters of American Yam Bean (Pachyrhizus erosus L.) under Saline Condition. Journal of Experimental Agriculture International 44(4):45-58. |
|
|
Pauwels JM, Van Ranst E, Verloo M, Mvondo ZA (1992). Analysis Methods of Major Plants Elements. Pedology Laboratory Manual: Methods of plants and Soil Analysis. Stock Management Equipment of Worms and Chemical Equipment. AGCD, Agriculture Publications: Brussels pp. 1-28. |
|
|
Pontigo S, Ulloa M, Godoy K, Nikolic N, Nikolic M, Luz Mora M, Cartes P (2018). Phosphorus efficiency modulates phenol metabolism in wheat genotypes. Journal of Soil Science and Plant Nutrition 18(3):904-920. |
|
|
Scagel CF, Bryla DR (2017). Salt exclusion and mycorrhizal symbiosis increase tolerance to NaCl and CaCl2 salinity in 'Siam Queen' basil. HortScience 52(2):278-287. |
|
|
Shahriaripour R, Tajabadipour A, Mozaffari V (2011). Effects of Salinity and Soil Phosphorous Application on Growth and Chemical Composition of Pistachio Seedlings. Communications in Soil Science and Plant Analysis 42(2):144-158. |
|
|
Shapiro SS, Wilk MB (1965). An Analysis of Variance Test for Normality (Complete Samples). Biometrika 52(3/4):591-611. |
|
|
Somawathi KM, Rislya V, Wijensinghe DGNG, Madhujith WMT (2015). Antioxidant Activity and Total Phenolic Content of Different Skin Coloured Brinjal (Solanum melongena). Tropical Agricultural Research 26(1):152-161. |
|
|
Taïbi K, Taïbi F, Abderrahim LA, Ennajah A, Belkhodja M, Mulet JM (2016). Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. South African Journal of Botany 105:306-312. |
|
|
Tang H, Nui L, Wie J, Chen X, Chen Y (2019). Phosphorus Limitation Improved Salt Tolerance in Maize Through Tissue Mass Density Increase, Osmolytes Accumulation and Na+ Uptake Inhibition. Frontiers in Plant Science 10:856. |
|
|
Yang X, Liang Z, Wen X, Lu C (2008). Genetic engineering of the biosynthesis of glycine betaine leads to increased tolerance of photosynthesis to salt stress in transgenic tobacco plants. Plant Molecular Biology 66(1):73-86. |
|
|
Yang X, Wang X, Wei M (2010). Response of photosynthesis in the leaves of cucumber seedlings to light intensity and CO2 concentration under nitrate stress. Turkish Journal of Botany 34(4):303-310. |
|
|
Yemm EW, Cocking EC (1955). The determination of amino acids with ninhydrin. Analyst 80(948):209-214. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0