ABSTRACT
Acute toxicity and cytotoxicity of ethanol extract of Samanea tubulosa (EESt) pods were evaluated in Swiss mice. Acute toxicity studies were conducted based on OECD guidelines 420, where the limit test dose was 5000 mg/kg. Observation was made and recorded systemically for 1, 2, 4 and 24 h after the administration of dose for skin changes, morbidity, aggression and sensitivity of the behavior of the animals. For the cytotoxicity, 3-[4,5-dimethylthiazol-zil] -2,5-diphenyltetrazolium (MTT) test and hemolysis were performed with concentrations of 6.25 to 800 µg/ml. No significant variation (p < 0.05) in the body and organ weights between the control and the treated group was observed after 14 days of treatment. Pathologically, neither gross abnormalities nor histopathological changes were observed. No mortality was recorded in 14 days. Moreover, both cytotoxic tests made no significant alterations to be able to display the evidence of the effect of cytotoxic. Therefore, we suggest that EESt use is safe in a systemic and cellular level.
Key words: Samanea tubulosa, toxicity, 3-[4,5-dimethylthiazol-zil] -2,5-diphenyltetrazolium (MTT), hemolysis, natural product.
Medicinal plants have biological activities that are bene-ficial to humans because they contain certain compounds that have useful properties. However, the potential toxicity of their bioactive substances has not been well established (Rosidah et al., 2009). Long-term use without any evidence of risk may indicate that a medicine is harmless. However, the absence of any reported or documented side-effects is not an absolute declaration of the safety of herbal medicines (Who, 2000). Detailed toxicological evaluation has to be performed using suitable experimental animals to supply guidelines for selecting a ‘safe’ dose for human and animal use. Tests to assess toxicity are performed to classify and label substances appropriately according to their lethality or toxicity potential as prescribed by law (Valadares, 2006). They are conducted to know the conditions in which the chemicals have toxic effects, the nature of these effects and the safe levels of being exposed to them (Júnior and Borges, 2012). Among the medicinal plants, Samanea genus, that has been studied a little, has a promising biological potential for having rare therapeutic applica-bility in scientific research. Botanical studies highlight the existence of some species such as Samanea saman, Samanea inopinata and Samanea tubulosa, represen-tatives of this genus. There are few morphological and anatomic differences between S. saman, S. innopinada and S. tubulosa. The basic difference between S. tubulosa and S. inopinata is observed in the fruiting; while S. saman is different from S. inopinata and S. tubulosa in structures, like leaflets and bark texture (Zapater et al., 2011; Durr, 2001). S. tubulosa is known in Piauí, Alagoas, Maranhão, Paraíba, Pernambuco and Sergipe as “bordão-de-velho”; in Bahia as samaneiro and sete cascas, Ceará. This species belongs to the family Leguminosae (Mimosoideae); it is a large tree that can reach 4 to 18 feet and 25 to 45 cm diameter. The fruit pods are sessile and indehiscent; and 10 to 18 cm long, with 20 to 30 seeds. It has wide applicability in animal and human feed; its fermented fruit can be used for ethanol production and apiculture with flowers. Its pods are edible and the pulp is sweet (Lorenzi and Souza, 1995; Carvalho, 2007).
This study was conducted at the Federal University of Piauí. Ethanol extract of S. tubulosa administered on female Wistar rats was toxic to the fetus, causing malformations, fetal resorption and pronounced decrease in birth weight (Sales et al., 2011). Due to these results, we aimed to evaluate the toxicity in vitro and in vivo of ethanol extract (EESt) pods of S. tubulosa.
Preparation of the extract
The ethanolic extract of the pods was prepared from dry pods in an oven at 45°C. It was subsequently ground in a mill and subjected to electric trituration with 99.5% ethanol for seven days at room temperature; and then protected from light. The extract was concentrated in a rotary evaporator at 50°C, packed in amber glass bottles and kept in the refrigerator and subsequently lyophilized.
The pods of S. tubulosa were collected from Teresina-PI, Center of Agricultural Sciences, Federal University of Piauí (UFPI). They were identified in the Herbarium Graziela Barroso- UFPI, where a voucher specimen was deposited under TEPB number – 27.261.
Animals
Female mice (Mus musculus, Swiss variety; 25 to 35 g) were used. They were reared in Maintenance of Animals Intended for Experimentation Department of Veterinary Morphophysiology - CCA/UFPI. The animals were housed in standard cages and kept at 24 ± 1°C and 12 h light dark cycle with water and food (FRI-LAB Rats - Fri-Ribe) ad libtum. Experimental protocols used are in accordance with the International standards and were approved by the Ethics Committee on Animal Experimentation of the Federal University of Piauí (CEEA/UFPI) protocol number 025/14.
Cytotoxicity assay in vitro of EESt
The macrophages used in this study were obtained from the peritoneal cavity of Swiss mice of four to five weeks. The removal of macrophages was performed in a laminar flow, in which the animals after euthanasia were kept in a supine position in a plate. 8 mL of phosphate buffered saline (PBS – NaCl 145 mM, Na2HPO4 9 mM, Na2HPO4 1 mM, pH 7,4) was administered to them and sterilized at 4°C in the abdominal cavity. After it was performed, the abdominal region was massaged gently, and aspiration of PBS injected using a sterile syringe attached to a needle was held. The material obtained was transferred to a conical polystyrene tube with a capacity of 50 ml, and centrifuged at 1000 rpm for 10 min. Shortly after, it was washed three times with PBS at 4°C. To investigate cell viability, it was counted in a Neubauer chamber diluted in Trypan blue dye.
We evaluated the cytotoxicity of ethanol extract of S. tubulosa using the test of bromide 3-[4,5-dimethylthiazol-zil] -2,5-diphenyltetrazolium (MTT) (Sigma-Aldrich, St. Louis, EUA). To a 96 well plate, 100 μl of supplemented RPMI 1640 and 2 x 105 macrophages were added. For cell adhesion, these cells were incubated at 37°C and 5% CO2 for 4 h. After that time, supplemented RPMI media used for the removal of cells that did not adhere were washed twice. 100 μl of supplemented RPMI 1640 with diluted EESt (800 to 6.25 µg/ml) was added later. They were then incubated for 48 h at the end of the incubation.10 μl of supplemented RPMI 1640 with EESt was added to MTT diluted in PBS (5 mg/ml). The samples were incubated for 4 h in an oven at 37 with 5% CO2, and then the supernatant was discarded. Then, 100 μl DMSO was added to all the wells. Then, the plate was placed under stirring for about 30 min in an agitator Kline (AK model 0506) at room temperature to complete dissolution of the formazan. Finally, the reading was performed at 550 nm in a Biotek reader (model ELx800) plate. The results were expressed in percentage and mean cytotoxic concentration (CC50); the control group was taken as 100% (Nogueira et al., 2007). The negative control was given supplemented RPMI 1640 media 0.2% DMSO.
Acute toxicity of EESt
This procedure followed the Acute Oral Toxicity protocol recommended by OECD 425 (OECD, 2001). The animals were divided into five groups, with six animals each. They were treated orally with a single dose of the compound dissolved in distilled water and increasing doses of 2000, 3000, 4000 and 5000 mg/kg in 10 ml/kg volume. The control group received distilled water as a single dose, orally, in 10 ml/kg volume. All animals were observed after treatment, every 30 min during the first 4 h and daily until day 14. The parameters evaluated were: death, alertness, sedation, ptosis, dyspnea, urination, diarrhea, convulsions, spontaneous motor activity, postural reflex, piloerection, response to touch, among others. The total number of deaths in each group was quantified by the end of the period. The animals were then anesthetized by anesthetic combination (50 mg/kg ketamine + 5 mg/kg xylazine) for blood collection and then euthanized by overdose of the same anesthetic combination for the removal of organs.
Cytotoxicity in human erythrocytes
For evaluation of the hemolytic activity, human red blood cells type O+ blood was collected in anticoagulant (EDTA). After collection, the erythrocytes were diluted in 80 μl PBS, adjusting the concentration of blood to 5% of erythrocytes. Then, EESt was added (800 to 6.25 mg / mL), and diluted in a volume of 20 μl PBS. After this procedure, it was incubated for 1 h at 37°C and the reaction was stopped by adding 200 μL of PBS. Then, the suspensions were centrifuged at 1000 g for 10 min at room temperature. The supernatant was subjected to spectrophotometry at a wavelength of 550 nm to quantify the hemolytic activity. The 0 (negative control) and 100% hemolysis (positive control) was determined by replacing the sample solution tested with an equal volume of PBS and sterile Milli-Q water, respectively. The results were expressed in percentage and average concentration in hemolytic (CH50), considering the positive control as 100% hemolysis (Löfgren et al., 2008).
Statistical analysis
All assays were performed in triplicate and in three independent experiments. Analysis of variance (ANOVA) followed by a post-hoc Newman-Keuls and Tukey test was performed, taking a P value of < 0.05 as the minimum level required for statistical significance.
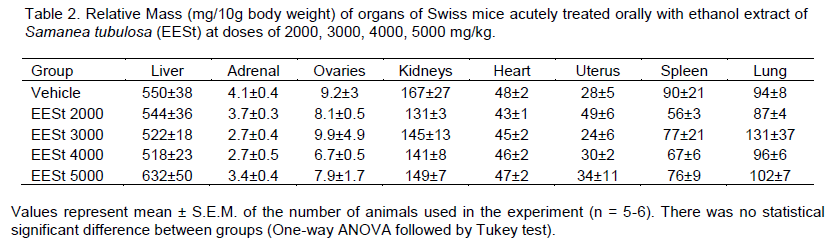
Tests assessing acute systemic toxicity are used to classify and label substances appropriately according to their lethality or toxicity potential as prescribed by law. Besides the lethality, other parameters are investigated in studies of acute systemic toxicity to identify toxicity potential in specific organs, identify toxicokinetics and dose-response relationship (Valadares, 2006). Toxico-logy studies are designed to evaluate the erroneous idea that herbal products are not toxic or do not have adverse effects because they are natural, and that the popular use of medicinal plants serves as validation for the effectiveness of such drugs (Simões et al., 2004). In the experimental model recommended by OECD 425 (OECD, 2001), acute treatment with distilled water and ethanol extract of S. tubulosa (EESt) at doses of 2000, 3000, 5000 mg/kg did not cause death in animals. Evidence shows that only one death was in the group treated with 4000 mg/kg (Table 1). The calculation of LD50 could not be performed because no toxicity was observed in the tested concentrations. This shows that it is safe as the administration route used and the doses studied. This supports the use of EESt in several experimental trials. Macroscopic analysis of liver, adrenal, ovaries, kidneys, heart, uterus, spleen and lung of Swiss mice treated orally with EESt at the doses recommended in this study showed no changes in their color and morphology. This indicates that the extract was not able to produce structural changes in these organs. This corroborates the data in Table 2 that there was no significant difference between the tested groups regarding the relative organ weights. Several observa-tions were
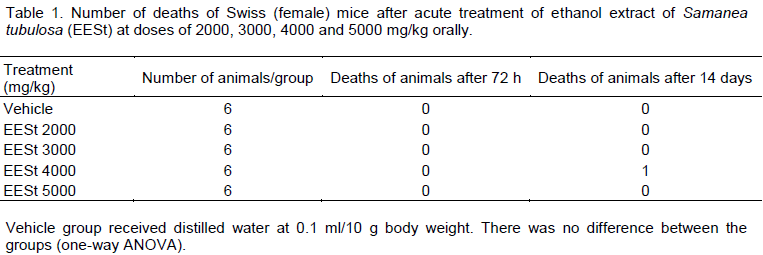
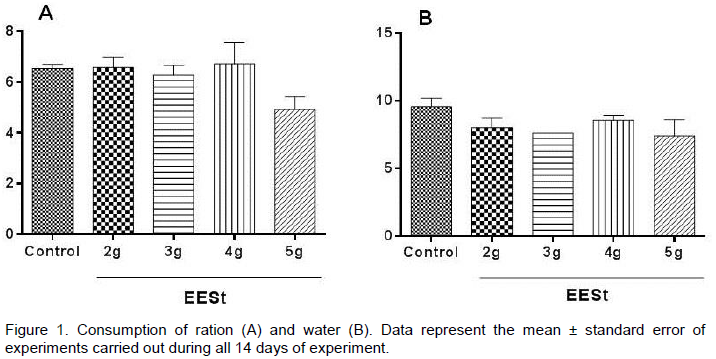
made when administering the EESt, such as alertness, sedation, dyspnea, urination, diarrhea, convul-sions, voluntary motor activity (ptosis) and automatic (postural reflexes), piloerection, response to tactile stimuli, the presence of death, which were not observed in any change between the groups tested. Figure 1 shows that the EESt tested in different doses (2, 3, 4 and 5 g) did not differ significantly regarding the consumption of food and water between the groups.
Another very important test in our study was the in vitro cytotoxicity (MTT/Hemolysis), where it is in a biological system that materials have direct contact with cell cultures in order to analyze their direct effect on cells (Christina et al., 2009). Although the results of the tests that assess cytotoxicity in vitro may not have a direct correlation in vivo, it is safe to say that if a material induces demonstrably a cytotoxic reaction in tests involving cell culture, it is very likely to develop toxicity when applied to living tissue (Osorio et al., 1998). Figure 2 shows that in our assay at different concentrations (from 6.25 to 800 µg/ml), the extract did not degenerate the cell membrane of macrophages, so there is no cytotoxicity in S. tubulosa. This is because it was not possible to calculate the value of CC50.
.png)
Tests performed with erythrocytes allow the evaluation of the potential of a drug to cause damage to the cell plasma membrane. This could be either by forming pores or rupturing totally, leading to cell damage or changes in membrane permeability (Costa-Lotufo et al., 2002). This model is used for a preliminary study of the effects of toxic substances; a possible indicator of damage to cells in vivo (Aparicio et al., 2005). The results of the hemolysis test with EESt (Figure 3) corroborate with previous results and the tested concentrations (6.25 to 800 µg/ml). EESt showed low toxicity with erythrocytes, producing no significant effect.
The results obtained through the various toxicological studies demonstrated that EESt has no acute oral toxicity at the doses studied, as there was no significant effect on the study of the cytotoxicity in macrophages and erythrocytes. Thus, we can suggest that its use is safe in systemic and cellular level of Swiss mice and human erythrocytes.
The authors declare no conflict of interest.
REFERENCES
|
Aparicio RM, Garcia-Celma MJ, Pilar VM, Mitijans M (2005). In vitro studies of the hemolytic activity of microemulsions in human erythrocytes. J. Pharmaceut. Biomed. 39(5):1063-1067.
Crossref
|
|
|
|
Carvalho PER (2007).Circular Técnica 132-Bordão-de-Velho, Samanea tubulosa. Embrapa. 132:1-6.
|
|
|
|
|
Christina V, Pavesi S, Wadt NS, Fernandes KP (2009). De arnica brasileira (Solidago microglossa) e arnica paulista (Porophyllum ruderale) 8(1):99-104.
|
|
|
|
|
Costa-Lotufo LV, Cunha GMA, Farias PAM et al.(2002). The cytotoxic and embryotoxic effects of kaurenoic acid, a diterpene isolated from Copaifera langsdorffii oleo-resin. Toxicon. 40(8):1231-1234.
Crossref
|
|
|
|
|
Durr PA (2001). The biology, ecology and agroforestry potential of the raintree, Samanea saman (Jacq.) Merr. Agrofor. Syst. 51 223-237.
Crossref
|
|
|
|
|
Júnior H, Borges L (2012). Avaliação da toxicidade aguda do extrato hexânico de frutos de Melia azedarach (Meliaceae) em camundongos. Ciência Animal. 13(4):512-519.
|
|
|
|
|
Löfgren SE, Miletti LC, Steinde ML, Bachére DE, Barraco MA (2008). Trypanocidal and leishmanicidal activities of different antimicrobial peptides (AMPs) isolated from aquatic animals. Exp. Parasitol. 118:197-202.
Crossref
|
|
|
|
|
Lorenzi H, Souza HM (1995). Plantas ornamentais no Brasil: arbustivas, herbáceas e trepadeiras. Editora Plantarum, São Paulo.
|
|
|
|
|
Nogueira IAL, Leão ABB, Vieira MS, Benfica PL, Bozinis MCV (2007). Efeito Citotóxico do Synadeniumum bellatum. Revi. Eletr. Farm. 2:50-53.
|
|
|
|
|
OECD (Organisation for Economic Co-operation and Development) (2001). Guidelines for the Testing of Chemicals.OECD 425 Acute Oral Toxicity—Modified Up and Down Procedure. Organisation for Economic Cooperation and Development, Paris.
|
|
|
|
|
Osorio RM, Hefti A, Vertucc IFJ, Shawley AL (1998). Cytotoxicity of endodontic materials. J. Endodont. 24:91-96.
Crossref
|
|
|
|
|
Rosidah YMF, Sadikun A, Ahmad M, Akowuah GA, Asmawi MZ (2009). Toxicology evaluation of standardized methanol extract of Gynura procumbens. J. Ethnopharmacol. 123:244–249.
Crossref
|
|
|
|
|
Sales PAB (2011). Toxicidade reprodutiva e sistêmica do extrato hidroalcoólico de vagens de Samanea tubulosa (Benth.) em ratas Wistar, Universidade Federal do Piauí-UFPI.
|
|
|
|
|
Simões CMO, Schenkel EP, Gosmann G, Mello JGC, Mentz LA, Petrovick P (2004). Farmacognosia: da Planta ao Medicamento. 5ed., Editora da Universidade Federal do Rio Grande do Sul, Porto Alegre, 1096.
|
|
|
|
|
Who (2000). General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine.In Annex II. World Health Organisation. 27-32.
|
|
|
|
|
Valadares MC (2006). Avaliação De Toxicidade Aguda: Estratégias Após a " Era Do Teste Dl 50. Revi. Eletr. Farm. 3(2):93-98.
|
|
|
|
|
Zapater MA, Hoc OS, Lozano EC (2011). El género Samanea (Leguminosae, Ingeae), novedad para la flora argentina. Darwiniana 49:7-15.
|
|