ABSTRACT
The bayoud is transmitted by the fungus Fusarium oxysporum f. sp. albedinis, which causes drying and rapid die back. To date, the disease has destroyed more than 12 million date palms in Morocco, or two-thirds of the producers of the best dates trees in this country, and three million of palm trees in Algeria with the threat of the spread of this disease to oasis southeast of Algeria. The research on this disease is very few in Algeria, in this case, this work had objective to study the growth and spread of the disease bayoud on the Algerian palm. is based on the observation of symptoms on palms, it showed that all regions with palm in Bechar (Saoura) and in Adrar (Touat), affected with some palm of Ghardaïa. Prophylactic measures are taken to protect and preserve our date resources (including 'Deglet Nour') in free palm (Zibans and Oued Righ in southeast of Algeria) by improving irrigation methods and the use of free releases, and determination date palm cultivars resistant to the fungus as Takarboucht in oasis of Ghardaïa and Adrar, Hmira and Hartan for Bechar’s date palms.
Key words: Date palm, bayoud disease, saoura, resistance, IPM.
The name Bayoud comes from the Arabic word, "abiadh", meaning white which refers to the whitening of the fronds of diseased palms. This disease was first reported in 1870 in Zagora-Morocco. By 1940, it had already affected several date plantations and after one century, the disease has practically affected all Moroccan palm groves, as well as those of the western and central Algerian Sahara (Killian and Maire, 1930; Toutain, 1965 ; Toutain 1972; Toutain and Louvet, 1972). Bayoud disease causes considerable damage that can sometimes take on spectacular proportions when the disease presents its violent epidemic aspect. Bayoud has destroyed in one century more than ten million palms in Morocco and three million in Algeria (Figure 1). Bayoud destroyed the world's most renowned cultivars that are susceptible to the disease and particularly those which produce high quality and quantity fruit (Medjool, Deglet Nour, BouFegouss or Feggouss). The result is an influx of farmers who have abandoned their land and moved to large urban centres (Toutain and Louvet, 1974). The continued spread of bayoud highlights the problem threatening the important plantations of Deglet Nour and Ghars in Oued Righ, Zibans in Algeria and even in Tunisia, which is presently free of the
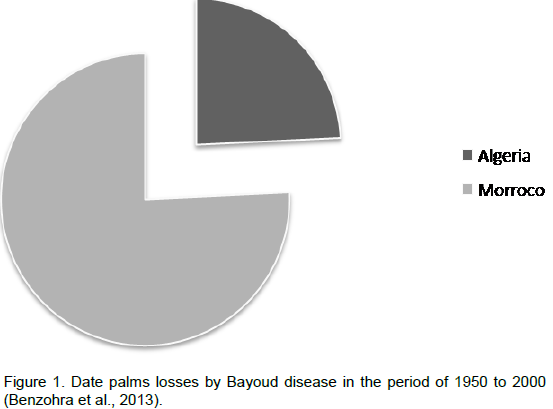
disease, but has 70 to 80% of the date palm areas under cultivars are susceptible to this disease (Meloncon, 1947 ; Dubost, 1972). The disease continues to advance relentlessly to the east, despite prophylactic measures and regular attempts at eradication undertaken in Algeria (Djerbi et al., 1985). It is evident therefore, that Bayoud constitutes a plague to Saharan agriculture and at the present expansion rate, it will certainly pose serious problems of human, social and economic nature to other date-producing areas of the world.
The bayoud disease attacks mature and young palms alike, as well as offshoots at their base (Saaidi, 1979). The first symptom of the disease appears on a palm leaf of the middle crown (Figure 2). This leaf takes on a leaden hue (ash grey colour) and then withens, from bottom to top, in a very particular way: some pinnae or spines situated on one side of the frond wither progressively from the base upward to the apex. After one side has been affected, the whitening begins on the other side, progressing this time in the opposite direction from the top of the frond to the base. A brown stain appears lengthwise on the dorsal side of the rachis and advances from the base to the tip of the frond, corresponding to the passage of the mycelium in the vascular bundles of the rachis. Afterwards, the frond exhibits a characteristic arch, resembling a wet feather and hangs down along the trunk. This whitening and dying process of the pinnae may take from a few days to several weeks. The same succession of symptoms then begins to appear on adjacent leaves. The disease advances ineluctably and the palm dies when the terminal bud is affected. The palm can die at any time from several weeks to several months after the appearance of the first symptoms (Figure 3). The rapid evolution of the symptoms depends mainly on planting conditions and on cultivar date palm. A small number of disease infected roots, reddish in colour, are revealed when an affected palm is uprooted. The spots are large and numerous towards the base of the stipe. As they advance towards the upper parts of the palm, the coloured conducting fascicles separate and their complicated path inside the healthy tissues can be followed. Palm fronds manifesting external symptoms exhibit a reddish brown colour when cut, showing highly coloured conducting fascicles. There is, therefore, a continuity of vascular symptoms that exist from the roots of the palm to the tips of the palm fronds. The observation of symptoms is necessary to recognise the bayoud, but to identify this disease with certainty, samples of affected fronds must be analysed by a specialised laboratory.


The causal organism responsible for bayoud is a microscopic fungus which belongs to the mycoflora of the soil and is named Fusarium oxysporum forma specialis albedinis (Killian and Maire, 1930; Malencon, 1934). The first precise descriptions of the fungus were done by Malençon (Malencon, 1950a; b), Pereau-Leroy (Pereau-Leroy, 1958) and more recently completed by the works of Bulit et al. (1967) and Louvet et al. (1970). The causal organism responsible for Bayoud is a microscopic fungus which belongs to the mycroflora of the soil. It belongs to the group Fungi lmperfecti, the Order Moniliales, the Family Tuberculariacae and, at the present time, is named F. oxysporum forma specialis albedinis (Malencon) Snyd. and Hans.
Macroscopic characteristics
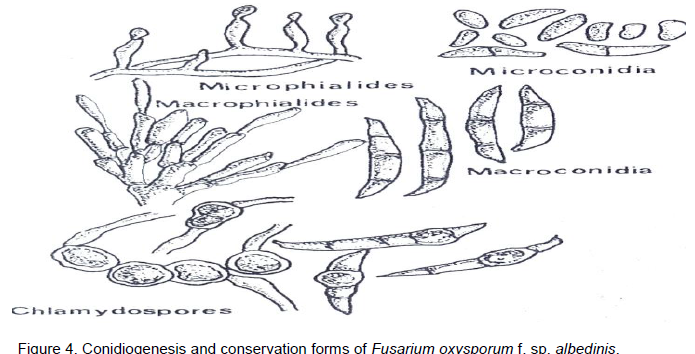
The isolation, the characterisation, and the maintenance of the F. oxysporum f. sp. albedinis culture has been the subject of numerous studies (Snyder and Hansen, 1972). Although, it is easy to obtain F. oxysporum f. sp. a/bedinis cultures from rachis fragments of palm leaves which exhibit vascular symptoms, it is very difficult to preserve the original type of colony (which is still called the wild type). Indeed, the wild form shows greatly unstable cultural characteristics under normal conditions and rapidly produces mutants. In culture, F. oxysporum f. sp. albedinis forms a fine, clear and curly mycelium in which small orange-pink sporodochia are produced (Figure 4). Blue to black sclerotia sometimes are born in the medium. They are either dispersed in the mycelium or sometimes form groups.
Microscopic characteristics
F. oxyysporum f. sp. albedinis occurs as a septate hyaline mycelium. It is fine and uniform in young cultures, exhibiting, however, in older cultures hypertrophic cells that occur in chains; they are round, greatly resembling chlamydospores, but without thickening of the wall. Asexual multiplication occurs by microphalides swollen at the base and pointed at the tip, arising perpendicularily from the mycelium. At the apical tip of these phialides, microconidia (microphialospores) form continuously and endogenetically, each one being pushed by the next and they adhere together in the form of small moist heads. Great numbers of hyaline microconidia, varying in form and dimensions, can be found in the same culture (3 to 15 x 3 to 5 µm). In young cultures they are globulous, while in the older cultures they are more elongated. Microconidia are often unicellular, sometimes bicellular but rarely have two septa. Macrophialides, larger than microphialides, group together to form sporodochia and more rarely pionnotes (Figure 4). In culture, F. oxysporum f. sp. albedinis also produces a few macroconidia with pediform base and short, pointed tips; most have three septa, though some have four or five and measure 20 to 30 x 3 to 5 µm (Figure 3). In older cultures, or in an agar medium covered over with earth, F. oxysporum f. sp. albedinis forms chlamydospores that are uniform and globulous, with a smooth thick wall varying from 6 to 20 µ (Baysal et al., 2010). They may be either intercalary or terminal and are isolated or grouped in two or four in short chains. They are formed either on the mycelium or from macroconidia (Figure 4). Sclerotia are dark blue-black, measure about 1 mm in diameter and occur rarely (Sedra and Djerbi, 1985).
Physiologic characteristics
The physiology of F. oxysporum f. sp. albedinis has been little studied. Malencon (1947) determined the optimum temperature range for growth. Growth begins at 7°C, remains slow until 12°C, becomes more rapid between 21 and 27.5°C and stops at 37°C (Shabani and Kumar, 2013; Shabani et al., 2014). Louvet and Bulit (1978) reported that the capacity of the fungus to use complex carbon sources and to develop in the presence of high concentrations of carbonic gas or penta-chloro-nitro-benzene. Bounaga (1975) found that the best mycelium growth occurs at 28°C. The marked preference of F. oxysporum f. sp. albedinis for pectin, mannose, xylose and cellulose was also noted and in addition to that organic nitrogenous sources are better metabolised than mineral nitrogen. The influence of sodium chloride was also studied and it was observed that there is no lessening of growth up to a concentration of 40 g/l. This observation is similar to Toutain and Louvet (1972) who found that Bayoud advances. Normally in salty fields, Dubost et al. (1970) also studied the pectinolytic and celluloytic enzymes of the fungus. These authors showed the important difference between the enzymatic activities of different isolates of F. oxysporum f. sp. albedinis, but they did not establish a correlation between this activity and the pathogenic power of the fungus.
F. oxysporum f. sp. albedinis is preserved in the form of chlamydospores in the dead tissues of infected palm, especially in the roots which have been killed by the disease and in the soil. A contamination occurs regularly from palm to palm and more rapidly as the amount of irrigation increases. The appearance of the disease in locations far from the original infected area is caused primarily by the transport of infected offshoots or palm fragments harbouring the fungus. Many plants are often grown as intercrops in palm groves, notably lucerne (Medicago sativa L.; alfalfa), henna (Lawsonia inermis L.) and vegetables (Djerbi et al., 1985). These plants can harbour the bayoud organism without manifesting any symptoms (symptomless carriers) (Bengyella et al., 2012).
Survival, infection and disease cycle
Like all vascular oganisms of telluric origin, F.oxysporum f. sp. albedinis is preserved in the form of chlamydospores in the dead tissues of infected palm trees, especially in the roots of trees killed by it. With subsequent disintigration of such tissues, the chlamydospores may be released into the soil where they remain in a dormant state. The fungus is found at a depth varying from 5 to 30 cm, and sometimes deeper; it can be preserved for a long period of time, even when the palm trees have long since died (eight years or more). If nutrients reach the chlamydospores in sufficient quantity, the spore germinates and invades a root, entering the vascular tissues as a parasite (de la Perrière and Benkhalifa, 1991). Once the pathogen is inside the vascular element, it grows rapidly and the mycelium advances up the root and into the stem (Ghaemi et al., 2011; Laurence et al., 2012). The mycelium produces microconidia in the vessels and these are carried upwards by the water stream. When they flow up the vessel is impeded by a cross wall, the microconidia germinate, the germ tubes penetrate the wall and then microconidia formation is resumed on the other side of the wall. These new microconidia are in turn carried along to the next transverse wall in the same manner as that of the fusariose in the banana (Sedra and Djerbi, 1986). This process thus continues upward, internally through the tree to its terminal bud, leading to the death of the date palm. During the course of its upward progression, F. oxysporum f. sp. albedinis breaks out of the xylem and colonizes the surrounding parenchyma tissues of the tree by an inter-and intra-cellular mycelium which gives the tree the reddish brown colour that is characteristic of Bayoud. After the death of the date palm, the mycelium continues to develop in the parenchyma of the tree and forms numerous chlamydospores in the sclerenchyma cells (Sutherland et al., 2012). These constitute very favourable conditions for the survival of the fungus in the soil (Louvet, 1977; Dubost and Kada, 1974; Dubost and Kada, 1975).
Spread of Bayoud in palm groves
Little research has been done on the speed of Bayoud's advancement in a plantation. The best information comes from the experimental palm grove of Nebch at Zagora, Morocco (Toutain, 1970) and from the palm grove of In Salah in Algeria (Kada and Dubost, 1975) (Figures 5 and 6). Starting from a few affected trees (primary centres) Bayoud expands many foci; this is particularily visible in homogeneous plantations. Indeed, the contamination occurs regularily from tree to tree and more rapidly as the intensity of irrigation increases. In this case, the disease takes on an epidemic aspect; the number of affected trees increases rapidly and the life span of the diseased tree decreases. Thus, at Zagora, on a plot of land containing 125 palm trees of the susceptible Bou Feggous variety. This primary infection, began in 1956, has destroyed the whole plantation over a period of 14 years at the average rate of 6% per year. The intensive cultivation of palm groves, therefore, fosters the expansion of Bayoud. A high salt content of both soils and water (5 g/1) does not prevent or slow down the spread of Bayoud (de la Perrière and Benkhalifa, 1991). Periods of drought, accompanied by lack of water, result in a regression of the disease which then becomes dormant. On the other hand, as soon as irrigation becomes significant and frequent, the disease reappears more violently and becomes gravely epidemic, particularily when there is a predominance of susceptible
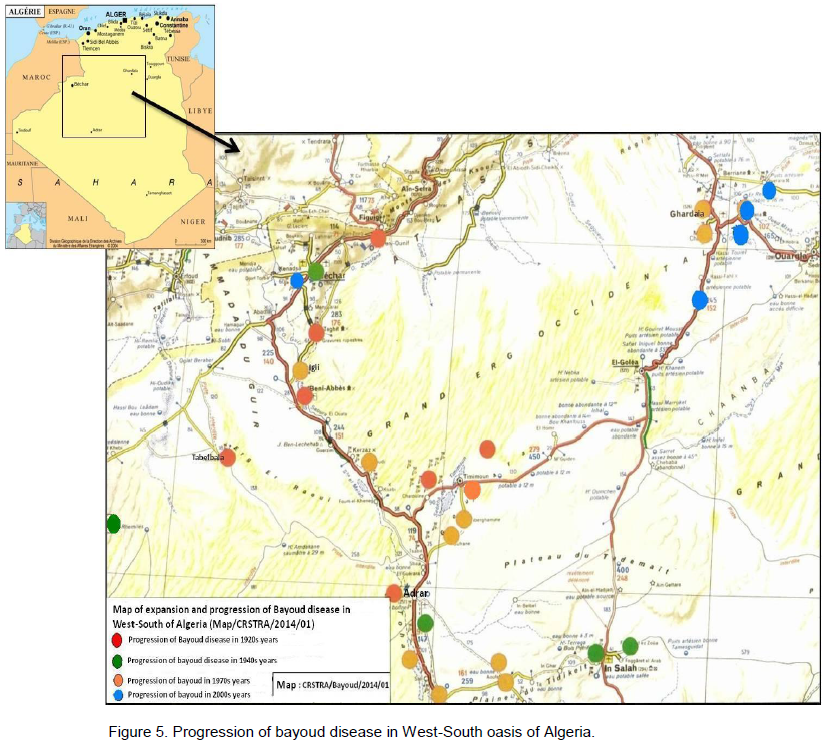
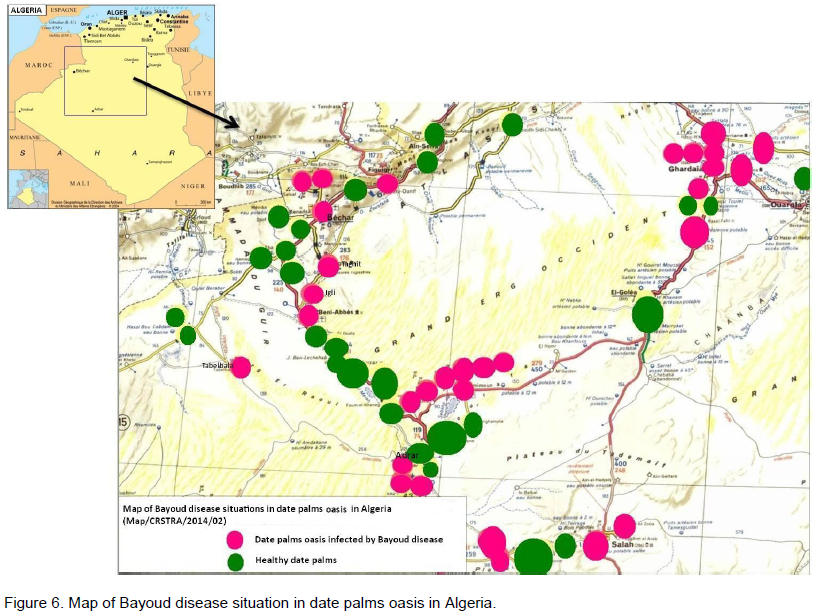
varieties. It thus seems that alternating dry and wet periods are favourable to an explosive expansion of the disease. This phenomenon has also been observed for other Fusarium diseases (Djerbi, 1970). Furthermore, according to Pereau-Leroy (1958), certain intercrops such as lucerne, alfalfa M. sativa, henna (L. inermis L.) vegetables … etc, foster strong attacks of Bayoud. The author thinks that this ellect is due to the influence of copious irrigation, indispensable to these crops during the hot season. A study by Laville and Lassois (1963) shows that the direction of the advancement of the disease in the Ain Salah palm grove is related to the dominant winds, irrigation and the salinity gradient. On the other hand, to date no work has been done to demonstrate the possible influence of nematodes on the spread of Bayoud. The appearance of foci that are rather far from the original focus is essentially related to the transport of infected offshoots or palm tree fragments harbouring the fungus. The consequences of the spread of Bayoud are particularity visible in Morocco. After the disappearance of a great number of the best varieties, presently in the Moroccan palm groves more than 50, of the trees are of seedling origin called Khalts, Sairs or Deguels (In arabic). These Sairs are often of bad quality, meaning of low commercial value.
Many plants are often grown among date palms in the groves, notably alfalfa, henna, etc. To date, F. oxysporum f. sp. albedinis has only been isolated from henna (Lawsonia inermia); indeed, the plant harbours the Bayoud organism without manifesting any symptoms. More recently in France, Mercier and Louvet (1973) also isolated the causal organism of Bayoud on Phoenix canariensis, another species of palm trees, which manifests the same symptoms as the vascular fusariose of the date palm trees.
CONTROL OF BAYOUD DISEASE
Chemical control
Soil treatment of this type of disease is destined, a priori, to fail and should therefore be avoided. Chemical control can, however, be feasible in the event of the discovery of primary sources of infection in a healthy area. In this case eradication techniques should be used: palms are uprooted and incinerated on the spot. The soil is then treated with methyl bromide or chloropicrin and the area closed off with replanting prohibited until further notice.
Cultural control
Since the factors that favour high yield in date palms (irrigation, fertilisation, etc.) are the same that favour the growth of the fungus, cultural techniques are not advised. However, a significant reduction in the amount of irrigation can retard the advance of infection, that is, stopping irrigation between the months of May and October, during the hot season in the northern hemisphere (Pereau-LeRoy, 1958; Dubost and Rellon, 1974). Since the contamination occurs mainly by root contact, disease-free palms can be isolated by digging a trench of 2 m deep around them. Water should be provided by a trough bridging the rest of the grove to this isolated plot. Under these conditions these palms can be protected for more than 10 years (Djerbi, 1983).
Prophylactic measures
The essential task is to prevent the movement of contaminated plant material from an infected palm grove to a healthy one. This material has been previously mentioned, consists mainly of offshoots, palm fragments, manure and infected soil, and artifacts made from these materials. Legislation preventing the conveyance of contaminated vegetative material from one country to another, or from one region to another, has been passed by various countries such as Algeria, Egypt, Iraq, Libya, Mauritania, Saudi Arabia, Tunisia and USA.
Genetic control
The only productive means of controlling bayoud disease lies in continued research into resistant varieties. Many resistant cultivars have already been obtained in Morocco from three sources: selection of bayoud-resistant varieties from those already existing (local and introduced), selection of high-quality, resistant clones from the natural population of the date palm, and creation of resistant and high quality varieties through a hybridisation programme (Djerbi et al., 1986). In addition, the present success of date palm propagation by in vitro culture will make it possible to rehabilitate the Moroccan and Algerian palm groves that have been destroyed by bayoud. It will also be possible to reconstitute the palm groves presently threatened by Bayoud and create new date-growing areas with the help of high quality, resistant varieties.
CONCLUSION AND PERSPECTIVES
The palm tree plays an important role in the ecology, economy and sociology of the Saharan environment; this tree is, in fact, irreplaceable in irrigable desert lands. Because of the considerable damage that Bayoud has caused in Moroccan, and Algerian palm groves and the threat that it constitutes to other countries, the disease has become the greatest enemy to date-growing regions of the world. As previously mentioned, the biological characteristics of F. oxysporum f. sp. albedinis and of its host make any type of successful chemical control unlikely. Furthermore, the projected prophylactic measures will never control Bayoud; they will only temporarily retard the spread of this disease. Genetic control remains the only solution to this problem. The first solution, leading to short-term (within 5 years) results. Consists of studying the date palm population existing in contaminated zones in order to select resistant varieties of good quality. This genetic potential will be complemented by the introduction of the world's best varieties which will then be tested for Bayoud resistance. There remains, however, the problem of propagation of selected cultivars. Indeed, the release of a resistant cultivar and its planting on a mass scale demands in turn the availability of large numbers of offshoots. It is obvious that the natural production of offshoots by palm trees is very slow and insufficient to fulfil the demand for offshoots in all areas damaged by Bayoud disease. The improvement of the technique of rooting light-weight offshoots in greenhouses equipped with "mist systems" will help to accelerate, at least slightly, the technique of vegetative propagation (Saadi, 1979). Nevertheless, this course of action does not solve the need for large number of offshoots.
The solution to the problem lies in the perfection of a method of date palm tissue culture. Several laboratories throughout the world are working on this subject and they have obtained encouraging results. Those of the labora-tories of Indio (USA) and Angers (France) are particu-larity interesting. Indeed, these authors have succeeded in obtaining young date palms from inflorescences or trom meristematic tissues. Once this method is perfected, intlnite numbers of highquality, Bayoud resistant date palms will be obtained from these clones. Thus, not only will it be possible to rehabilitate the Moroccan and Algerian palm groves that have been destroyed by Bayoud, but it will also be possible to reconstitute the palm groves presently threatened by Bayoud, as well as to create new date-growing areas with the help of high-quality, resistant varieties.
In conclusion, bayoud disease is an epiphytic disease for which there is no known cure at present. Only preventive measures could protect healthy date plantations from this disease. Therefore, the following measures are imperative:
i) Forbid the introduction of offshoots and all other plant material (palm fragments, artifacts made from date material, manure and infected soil) originating from bayoud infected countries or regions.
ii) Forbid the import of seeds and unprocessed products of symptomless carriers such as Alfalfa (Lucerne) and Henna from bayoud-infected countries or regions.
iii) Adopt legislation preventing the conveyance of the above plant material.
iv) Immediately report cases where symptoms similar to the ones caused by the bayoud appear.
v) Information on bayoud and other major diseases and pests is necessary for the success of all above actions and must be available to all date growers.
The authors have not declared any conflict of interests.
REFERENCES
|
Baysal Ö, Siragusa M, Gümrükcü E, Zengin S, Carimi F, Sajeva M, Da Silva JAT (2010). Molecular Characterization of Fusarium oxysporum f. melongenae by ISSR and RAPD Markers on Eggplant. Biochem. Genet. 48: 524-537.
Crossref
|
|
|
|
Bengyella L, Pranab R, Yekwa E, Waikhom S (2012). The Farmers Cry: Impact of Heat Stress on Fusarium oxysporum f. sp. dianthi, Interaction with Fungicides. Asian J. Plant Pathol. 6: 19-24.
Crossref
|
|
|
|
|
Benzohra IE, Megateli M, Berdja R (2013) Progression et extension du Bayoud du palmier dattier en Algérie. Proceeding Premier Séminaire International sur : les problématiques agronomiques en régions arides et semi-arides, 28 – 30th October 2013, university of Batna, Batna, Algeria.
|
|
|
|
|
Bounaga N (1975). Germination de microconidies et macroconidies de Fusarium oxysporum f. sp. albedinis. Bull. Soc. Hist. Nat. Afr. Nor. 66: 39 - 44.
|
|
|
|
|
De La Perrière RAB, Benkhalifa A (1991). Progression de la fusariose du palmier dattier en Algérie. Sécheresse, 02:119-128.
|
|
|
|
|
Bulit J, Bouhot D, Louvet J, Toutain G (1967). Recherches sur les fusarioses. I. Travaux sur le bayoud fusariose vasculaire du palmier dattier en Afrique du Nord. Annales des Epiphyties, 18 : 213-239.
|
|
|
|
|
Djerbi M (1970). Etude ecologique des actions parasitaires des espèces fusariennes a l'egard du blé. Bull. lnst. Agro. Tunisie No. 26-27: 5-58.
|
|
|
|
|
Djerbi M (1983). Diseases of the date palm Phoenix dactylifera. FAO, Baghdad, Iraq.
|
|
|
|
|
Djerbi M, Aouad L, Filali H, Saaidi M, Chtioui A, Sedra MH, Allaoui M, Hamdaoui T, Oubrich M (1986). Preliminary results of selection of high-quality bayoud-resistant clones among natural date palm population in Morocco. In: Proceedings of the Second Symposium on the Date Palm, Saudi Arabia, pp. 383-399.
|
|
|
|
|
Djerbi M, El Ghorfi A, El Idrissi Ammari MA (1985). Etude du comportement du henné Lawsonia inermis et de la luzerne Medicago sativa et quelques espèces de palmacées vis-à-vis du Fusarium oxysporum f.sp. albedinis, agent causal du bayoud. Annales de l'Institut National de la Recherche Agronomique de Tunisie, 58: 1-11.
|
|
|
|
|
Dubost D (1972). The bayoud disease in Algeria, history and diagnosis. In: Proceedings of the First International Seminar and Workshop on Bayoud, Algiers, Algeria, pp. 83-92.
|
|
|
|
|
Dubost D, Kada A (1975). Le bayoud à Ghardaia. Bulletin d'Agronomie Saharienne 3, 29-61.
|
|
|
|
|
Dubost D, Kada, A. (1974). Etude expérimentale de l'inoculation de jeunes plantules de palmier dattier par Fusarium oxysporum. Bulletin d'Agronomie Saharienne, 1 : 21-37.
|
|
|
|
|
Dubost D, Kellou R (1974). Organisation de la recherche et de la lutte contre le bayoud en Algérie. Bulletin d'Agronomie Saharienne, 1 : 5-13.
|
|
|
|
|
Dubost D, Kechacha L, Rether B (1970). Etude des enzymes pectinolytiques et cellulolytiques d'une souche monospore de Fusarium oxysporum f. sp. albedinis. AI Awamia, 35:195-211.
|
|
|
|
|
Ghaemi A, Rahimi A, Banihashemi Z (2011). Effects of Water Stress and Fusarium oxysporum f. sp. lycoperseci on Growth (leaf area, plant height, shoot dry matter) and Shoot Nitrogen Content of Tomatoes Under Greenhouse Conditions. Iran Agric. Res. 29: 51-62.
|
|
|
|
|
Kada A, Dubost D (1975). Le Bayoud à Ghardaia. Bull. Agr. Sahar. Alger. 01(03): 29-61.
|
|
|
|
|
Killian C, Maire R (1930). Le Bayoud, maladie du dallier. Bull. Soc. Hist. nat. Afr. 21: 89-101 (Abstr. Rev. Appl. Mycol. 10:99-100.
|
|
|
|
|
Laurence M, Burgess L, Summerell B, Liew E (2012). High levels of diversity in Fusarium oxysporum from non-cultivated ecosystems in Australia. Fungal Biol. 116: 289-297.
Crossref
|
|
|
|
|
Laville E, Lassois P (1963). Méthodes de Van der Planck et Jode de propagation du Bayoud. Fruits 18 (5): 249-253.
|
|
|
|
|
Louvet J (1977). Observations sur la localisation des chlamydospores de Fusarium oxysporum dans les tissus des plantes parasitees. Travaux de Diesil G. Viennot- Bourgin, I.N.R.A. Societe Française de Phytopathologic, pp. 193-197 (Paris).
|
|
|
|
|
Louvet J, Bulit J (1978). Some observations about Fusarium wilt of Date palms (Phoenix dactylifera L.). Pathological wilting of Plants, University of Madras, India, pp. 499-506.
|
|
|
|
|
Louvet J, Bulit J, Toutain G, Rieuf P (1970). Le bayoud, fusariose vasculaire du palmier dattier, symptômes et nature de la maladie, moyens de lutte. Al-Awamia, 35: 161-182.
|
|
|
|
|
Malençon G (1947). Mission d'étude dans les oasis du terri to ire d'Ain-Sefra et l'annexe de Tidikelt concernant une maladie du palmier dattier. Annis Inst. Agric. Alger. 3: 139-158.
|
|
|
|
|
Malençon G (1950a). La diffusion et l'épidémiologie de la maladie fusarienne du palmier dattier en Afrique du Nord. Revue Mycol.Suppl. Colon. 15: 45-60, Abstr. Rev. Appl. Mycol. 30: 154-155.
|
|
|
|
|
Malençon G (1950b). Le Bayoud, maladie fusarienne du palmier dattier en Afrique du Nord. Fruits d'Outre-mer 5: 279-289, Abstr. Rev. Appl Mycol. 30:155.
|
|
|
|
|
Malençon G (1934). Nouvelles observations concernant l'etiologie du Bayoud. In C.R. Hebd. Seanc. Acad. Sci. Paris, 196: 1259-1262 (Abstr. Rev. Appl. Mycol. 13: 505. Mercier S, Louvet J (1973). Recherches sur les Fusarioses. X – Une Fusariose vasculaire du Palmier des Canaries. Ann. Phytopathol. 5: 203-211.
|
|
|
|
|
Pereau-Leroy P (1958). Le palmier dattier au Maroc. Royaume du Maroc, Ministere de l'Agriculture, Institut Français de Recherches Fruitières Outre-mer, 142 p. Abstr, Rev. Appl. Mycol. 40: 236.
|
|
|
|
|
Saaidi M (1979). Contribution à la lutte contre le bayoud, fusariose vasculaire du palmier dattier. Ph.D. Thesis, University of Dijon, France.
|
|
|
|
|
Sedra MH, Djerbi M (1985). Mise au point d'une méthode d'identification in vitro du Fusarium oxysporum f. sp. albedinis, agent causal du bayoud. Annale de l'Institut National de la Recherche Agronomique de Tunis, No. 2, 58, 1-12.
|
|
|
|
|
Sedra MH, Djerbi M (1986). Comparative study of morphological characteristics and pathogenicity of two Fusarium oxysporum causing respectively the vascular wilt disease of date palm (Bayoud) and Canary Island palm. In: Proceedings of the Second Symposium on the Date Palm, Saudi Arabia, pp. 359-365.
|
|
|
|
|
Shabani F, Kumar L (2013). Risk levels of Invasive Fusarium oxysporum f. sp. albedinis in Areas Suitable for Date Palm (Phoenix dactylifera) Cultivation Under Various Climate Change Projections. PLoS ONE, 8: 483-404.
Crossref
|
|
|
|
|
Shabani F, Kumar L, Esmaeili A (2014). Future Distributions of Fusarium oxysporum f. spp. in European, Middle Eastern and North African Agricultural Regions under Climate Change. Agriculture, Ecosystems and Environment, 197: 96-105.
Crossref
|
|
|
|
|
Snyder CW, Hansen HN (1940). The species concept in fusarium. Am. J. Bot. 27(2): 64-67.
Crossref
|
|
|
|
|
Sutherland R, Viljoen A, Myburg AA, Van Den Berg N (2013). Pathogenicity associated genes in Fusarium oxysporum f. sp. cubense race 4. South Afr. J. Sci. 109: 01-10.
|
|
|
|
|
Toutain G (1972). Progression du Bayoud en palmeraies etablies sur terrain sales.AI-Awamia, 42: 65-75.
|
|
|
|
|
Toutain G, Louvet J (1972). Resistance to Bayoud in varieties of date palm. First International Seminar and Workshop on Bayoud, Algiers, October 1972, I.T.A.S. Alger: 208-210.
|
|
|
|
|
Toutain G (1970). Observations sur Ia progression d'un foyer actif de Bayoud ans une plantation reguliere de Palmier dattier. AI Awamia, 35: 155-161.
|
|
|
|
|
Toutain G (1965). Note sur l'épidémiologie du bayoud en Afrique du Nord. Al-Awamia, 15: 37-45.
|
|
|
|
|
Toutain G, Louvet J (1974). Lutte contre le bayoud. IV. Orientations de la lutte au Maroc. Al-Awamia, 53: 114-162.
|
|