Full Length Research Paper
ABSTRACT
The use of propolis for the treatment of various ailments in which inflammation and pain is involved is cross-cultural. This report was designed to investigate the analgesic and anti-inflammatory activities of propolis obtained from Gboko, Benue State using hexane, ethyl acetate, and ethanol solvents. Propolis sample obtained from beehives was homogenized, extracted and dried at 40°C. The dry extract was prepared in a solution of olive oil for pharmacological and toxicological tests. Acute toxicity of propolis in varying polarity was determined using albino rats. Oedema volume of albino rat’s paw was used to determine the anti-inflammatory activity of the sample. Analgesic property of the sample was investigated by measuring the number of acetic acid induced writhing in albino rats. Hexane, ethyl acetate, and ethanol extracts of propolis were non-toxic at 1,000 mg/kg bodyweight. During anti-inflammatory investigation, sample at 300 and 200 mg/kg bodyweight showed better activity than the standard drug, indomethacin. Best anti-inflammatory activity was observed with ethanol fraction at 300 mg/kg bodyweight. Best analgesic activity (100%) was recorded at 200 mg/kg in hexane. These results show that both non-polar and polar extracts of propolis have anti-inflammatory and analgesic activities at various dosages.
Key words: Propolis, anti-inflammatory, analgesic, acetic acid, diclofenac, indomethacin, Nigeria, polarity.
INTRODUCTION
A natural product is a chemical compound or substance produced by living organisms such as plants, animals, microbes, algae, and marine sponges. The term natural product has also been extended for commercial purposes to refer to cosmetics, dietary supplements, and foods produced from natural sources without added artificial ingredients. Several reasons such as knowledge of composition, structure and bioactivities of natural products necessitate the study of Natural Products (Nande and Igoli, 2017). Increasing evidence suggest that majority of the bioactive phytochemical components in plants impart physiological activities and may offer a variety of health benefits such as antioxidant, antibacterial, anti-inflammatory, and anticancer (Ipav et al., 2018) or anti-venom (Tor-Anyiin et al., 2015). Propolis is a natural product derived from plant resins and collected by honeybees (workers) to be used as glue and as draught-extruder for beehives (Khalil, 2006). Recent foray into bee propolis research has brought remarkable health benefits thereby making propolis a potential source for development of new drugs (Bankova et al., 2000). It has been reported that propolis contain at least 200 compounds with more than 100 being present in any given sample (Greenaway et al., 1999). These include fatty and phenolic acids and esters, substituted phenolic esters, bioflavonoids (flavones, flavanones, flavonols and others), terpenes, steroids, aromatic aldehydes and alcohols, and derivatives of sesquiterpenes, naphthalene and stilbenes (Marcucci et al., 1996).
Several research studies have corroborated the claim that, chemical composition and bioactivities of propolis depend on the phyto-geographical characteristics of the site of location which then varies with vegetation belts, countries, states and locality. Accordingly, these seasonal variations can influence the biosynthesis of plant metabolites and consequently affect the resin that is secreted by plants, which is used by the honeybees to produce propolis (Teixeira et al., 2008). According to Babatunde et al. (2015) and Khalil (2006), despite its sensitivity to geographical locations and the fact that constituent compositions differ according to each area, most propolis samples contain 50% resin, 30% wax, 10% essential oils, 5% pollen and 5% of other organic compounds in their chemical composition.
Due to the promise propolis holds, it has attracted researchers’ interest in the last decades because of its several biological and pharmacological properties including anti-inflammatory, analgesic, anticancer and antimicrobial activities (Christov et al., 2005). Several reports of the biological activities of propolis have been made in literature including antioxidant (Frozza et al., 2013), antimicrobial (Bispo-Junior et al., 2012), anticancer (Li et al., 2008), anti-inflammatory (Bueno-Silva et al., 2016; Cavendish et al., 2015), cytotoxicity (Alencar et al., 2007), repair of wounds (Batista et al., 2012) and antinociceptive (Cavendish et al., 2015) properties. Few reports are found on the propolis of Nigerian origin and none from Gboko.
In this paper, we report the analgesic and anti-inflammatory effect of extracts of propolis obtained from Gboko, Benue State using solvents of varying polarity; hexane, ethyl acetate and ethanol.
MATERIALS AND METHODS
Collection of propolis sample
Propolis sample was obtained from bee keepers in Gboko and the vegetation surrounding the hives was noted as Maranthes polyandra, Detarium microcarpum and Burkea africana.
Preparation of propolis extract
The homogenized propolis sample was placed in the thimble of the Soxhlet extractor and refluxed for 24 h with hexane, ethyl acetate and ethanol at a maximum temperature of 40°C. The liquid extract thus obtained was evaporated to dryness using a rotary evaporator at 40°C. The dried extracts of hexane, 12.68%, ethyl acetate, 7.46% and ethanol, 3.28% yield were prepared in a solution of olive oil for the pharmacological and toxicological tests.
Analgesic and anti-inflammatory activities of propolis sample
Experimental animals
Albino rats weighing between 70 and 120 g were obtained from the animal house of the Department of Chemical Sciences, University of Mkar, Mkar and used for the study. The experimental animals were kept in standard cages under standard conditions (25±2°C and a 12 h light/dark cycle) and maintained on standard pellets and drinking water ad libitum acclimatize for 5 days prior to commencement of the experiment. The animals were kept under hygienic conditions by constant cleaning and removal of faeces and spilled feeds from cages daily. The use and care of laboratory animals in the study were in accordance with ethical guidelines as contained in the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (EEC Directive 86/609/EEC) of 1986.
Acute toxicity studies
The acute toxicity (median lethal dose LD50) was determined using mice. Twenty five mice were grouped into five groups with five mice per group. The mice in each group were treated with propolis at doses of 100, 200, 300, 500 and 1000 mg/kg body weight .The experimental animals were observed for 72 h for signs and symptoms of toxicity (Arvouet-Grand et al., 1993).
Analgesic activity assay
Drug: Diclofenac sodium was used as a standard drug for the analgesic activity at a dose of 25 mg/kg body weight.
Acetic acid induced writhing test
Albino mice were induced with pain by intraperitoneal injection using 1% (v/v) acetic acid (2.3 mL/kg) as described by Santos et al. (1998). Animals were pre-treated with propolis sample prepared in olive oil at doses of 100, 200 and 300 mg/kg body weight orally, 45 min before acetic acid administration. Control animals received 2 mL volume of olive oil and the positive control animals were treated with the reference analgesic drug; diclofenac sodium (25 mg/kg). The number of abdominal constriction (writhings) was cumulatively counted over a period of 20 min after acetic acid administration. The percentage analgesic activity was calculated using the following formula (Santos et al., 1998).
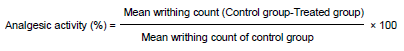
Anti-Inflammatory activity assay
Egg albumin induced oedema
The test was conducted using a modified method of Akah and Nwabie (1994). The rats were divided into five groups of three rats of either sex per group and were treated as follows: Group A which served as the negative control received olive oil (2 mL/kg bodyweight), while group B which served as the positive control received 25 mg/kg body weight of indomethacin, Groups C, D and E received 100, 200 and 300 mg/kg body weight of propolis orally, respectively. After 45 min of propolis sample administration, oedema was induced by sub-planter injection of 0.1 mL of fresh raw egg albumin in the left hind paw. Oedema volume was determined by measuring the paw volume using a thread. The readings were taken at 20 min intervals, that is, 0, 20, 40, 60, 80, 100, and 120 min after albumin administration.
The average increase in paw size of each group was calculated and compared with the control (olive oil) and indomethacin group.
Statistical analysis
The results were expressed as mean ± SEM. The statistical analysis was performed by means of Student’s t-test, whereas Analysis of Variance (ANOVA) followed by Dunnett’s multiple comparison test was used in order to compare more than two groups. P≤ 0.05 is indicative of significance.
RESULTS AND DISCUSSION
The use of propolis for the treatment of various ailments in which inflammation and pain are involved is cross-cultural and widespread. Its effect against diseases such as dermatological, odontological and gynaecological disorders has been widely reported (Castaldo and Capasso, 2002; De Castro, 2001). The mopping of free radicals generated by neutrophils during inflammation is the main mechanism of action for conventional anti-inflammatory drugs. It is also a well-known property of propolis (Paulino et al., 2003; El-Masry et al., 2011; Ichikawa et al., 2002). The process of inflammation includes production and release of mediators from neurons or damaged tissues which lead to various responses including pain (Paulino et al., 2003).
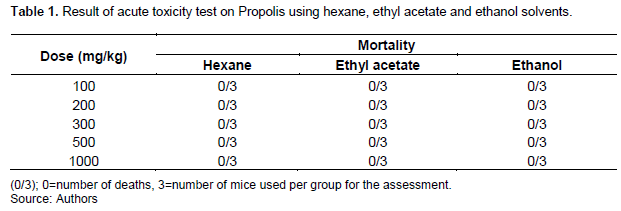
Hexane, ethyl acetate and ethanol extracts of propolis is non-toxic at 1000 mg/kg bodyweight (Table 1). This is in line with several reports of the relatively low toxicity of propolis. Arvouet-Grand et al. (1993) reported the oral LD50 of propolis extract to be greater than 7340 mg/kg in mice (Abozid and Ahmed, 2013). Dobrowolski et al. (1991) administered approximately 700 mg/kg orally to groups of 10 mice (five males and five females) and monitored them for 48 h. No deaths were recorded and the preparations were well tolerated by the test animals. In another study, Havsteen (1983) reports LD50 of flavonoids to range from 8000 to 40000 mg/kg bodyweight of rats. Considering the main component of propolis to be flavonoids, it should be relatively safe since flavonoids are of very low toxicity.
Anti-inflammatory activity
Figures 1, 2, 3, 4, 5 and 6 indicate that Propolis sample at 300 mg/kg bodyweight showed a steady increase in inhibition from 20 to 100 min. This result is better than the standard drug, indomethacin. The inhibitory effect of propolis at 200 mg/kg weight administration is also better than indomethacin. The best result was observed with 200 mg/kg bodyweight administration of sample. The inhibitory effect observed on the n-hexane extract of propolis is in a dose dependent manner. The higher the dosage, the more the inhibition. Inflammation is presumably initiated by the release of histamine, kinin, fibrinolysin and phospholipase A2 (Parandin and Daroogari, 2019). These intermediaries of inflammation induce oedema by vasodilation and increased vascular permeability (Zhang et al., 2015; Li et al., 2011; Xu et al., 2014; Uwaya et al., 2020). The anti-inflammatory effect of this sample may be due to the decrease in the amount of the induced intermediaries earlier mentioned.
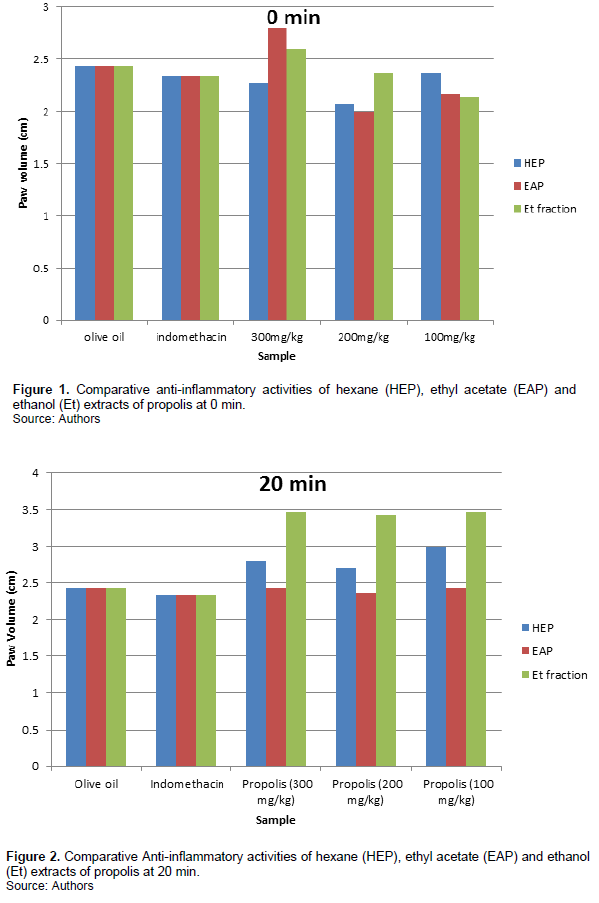
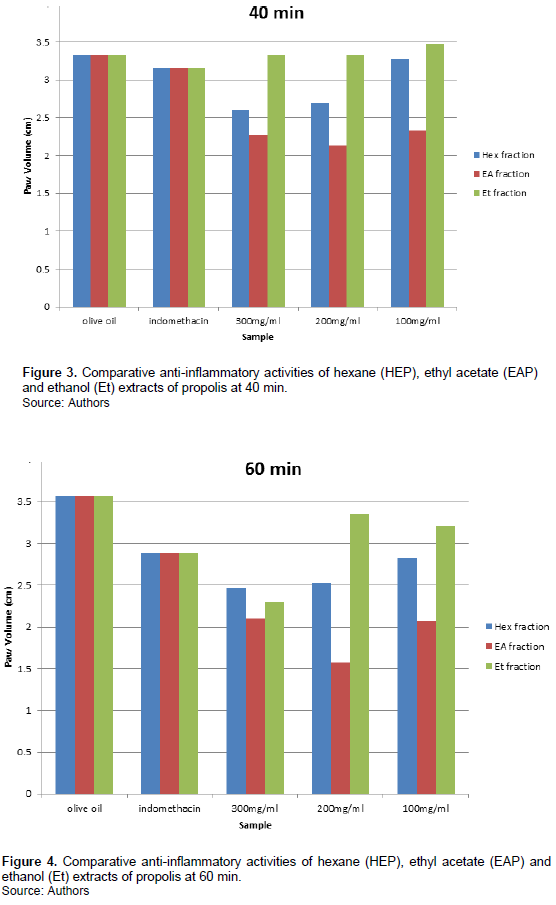
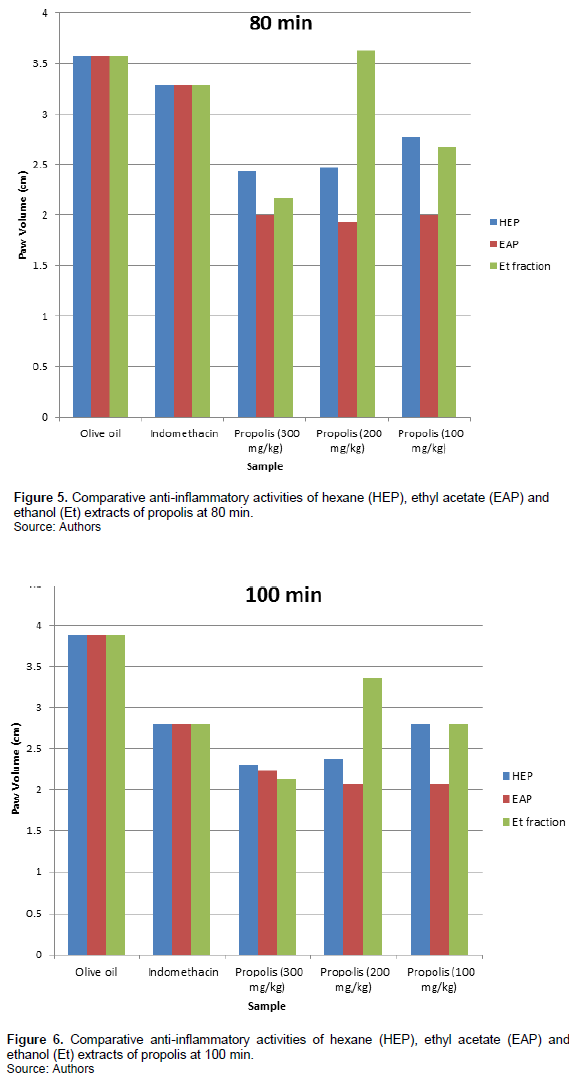
The inhibition of inflammation by ethyl acetate is not dose dependent. No regular pattern is observed with its administration. However, better anti-inflammatory activity is observed at 200 mg/kg bodyweight administration at 60 minutes as seen in Figure 4. This too is better than the standard drug, indomethacin.
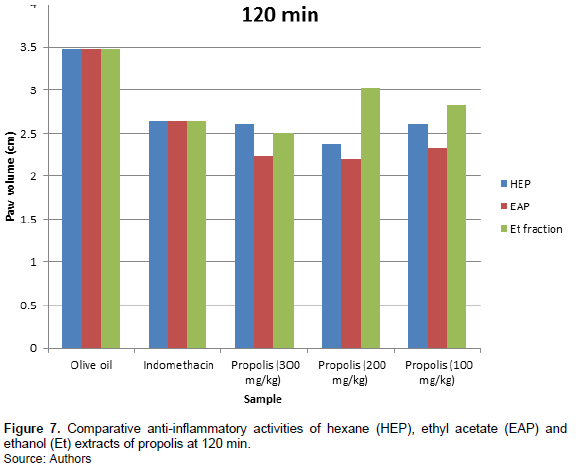
Figure 6 shows better inhibitory activity of ethanol fraction of propolis at 300 mg/kg bodyweight administration. The activity is time dependent. In the earlier phase, its activity at 300 mg/kg bodyweight administration is not as good as indomethacin. In the later phase; 60-120 min (Figures 4, 5, 6 and 7), the inhibition of inflammation is more pronounced and gets better than indomethacin.
Major constituents of propolis apart from resins and waxes include flavonoids, terpenoids and phenolic acids such as cinnamic acid, p-coumaric acid, caffeic acid, ferulic acid and their derivatives (Razmi et al., 2013; Pimenta et al., 2015; Sforcin, 2016). Parandin and Daroogari (2019) reported flavones, flavonols, flavanones, flavanonols, chalcones, dihydrochalcones, isoflavones, isodihydroflavones, flavans, isoflavones and neoflavonoids as types of flavonoids found in propolis. Several studies show that phytochemicals including flavonoids, phenolic acids and terpenoids have antimicrobial (Inui et al., 2014; Brodowska, 2017; Górniak et al., 2019), antinociceptive, anti-inflammatory and antioxidant properties (Murugan and Parimelazhagan, 2013; Wang et al., 2014; Yamanishi et al., 2014; Fazio et al., 2013; Parandin and Daroogari, 2019; Fernandez et al., 1998; Yonathan et al., 2006; Xu et al., 2016; Zhao et al., 2016). This is because they have been reported to prevent the production of inflammation intermediaries such as prostaglandins, arachidonic acid, histamine, bradykinins (Amresh et al., 2007; Mirazi and Hosseini, 2013), and cyclooxygenase 2 (Razmi et al., 2013) which are involved in inflammation and pain.
The solvent used during extraction determines the biological activity obtained (Devequi-Nunes et al., 2018). Similar biological activity may be more pronounced with increased polarity as concluded by Wieczynska et al. (2017), who reported a stronger antimicrobial activity of ethanolic extracts than hexane extracts of polished propolis. This improved anti-inflammatory activity of ethanolic extract of propolis obtained from Gboko may be as a result of increased availability of flavonoids and other phenolics due to the high polarity of ethanol.
Analgesic activity
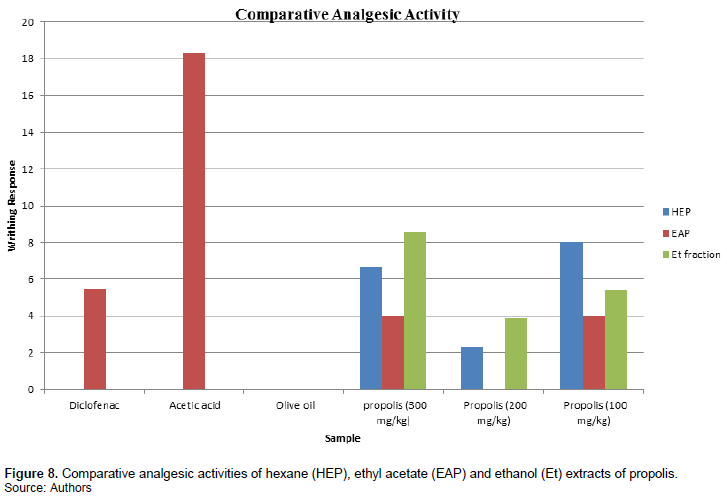
Hexane is highly selective during extraction. Figure 8 shows that hexane extract at 300 and 100 mg/kg bodyweight administration gave an analgesic activity of 78.14%. This result appears better than that of the standard analgesic used (diclofenac sodium). Best analgesic activity was recorded at 200 mg/kg bodyweight dosage which was 100% (Figure 8). This is not dose dependent.
Paulino et al. (2003) reported that the main constituents of propolis in temperate zones are flavonoids, whereas in the tropics other classes such as aromatic derivatives, specific terpenoids and prenylated p-coumaric acids and acetophenones may be found. In spite of these many variations in their chemical composition, they have several biological activities in common (Bankova et al., 1995; Seidel et al., 2008).
Bonvehi et al. (1994) reported poor result when determination and prediction of biological activity of propolis was based on analysis of individual compounds. This further proves the assertion that, biological activity of propolis is based on synergistic activity of individual compounds (Boisard et al., 2015; Chen and Shen, 2008).
Less activity was observed in ethyl acetate fraction. Figure 8 shows the standard drug, diclofenac had better activity than ethyl acetate fraction at 300 and 100 mg/kg dosages. The best analgesic activity was recorded at 200 mg/kg administration as seen in Figure 8. The analgesic effect of propolis obtained from Gboko against acetic acid induced writhing may be due to local peritoneal receptors or by inhibition of prostaglandin synthesis or activity (Adzu et al., 2001; Atta and Alkofahi, 1998).
Figure 8 shows that at 300 mg/kg dosage, ethanol fraction of propolis gave a relatively poor analgesic activity compared to 200 and 100 mg/kg dosage and diclofenac sodium. The component of propolis extracted, are more polar at this stage considering the high polarity of ethanol.
According to Ristivojevi? et al. (2015), non polar fractions of propolis contain mostly waxes and hydrocarbons which include alkanes, alkenes, alkadienes, monoesters, diesters, aromatic esters, fatty acids and steroids which in most cases do not exhibit any significant pharmacological activity. The observed activity of the n-hexane fraction may be due to a synergistic activity of the non polar components of the propolis. This is in line with the report of Ristivojevi? et al. (2015) that no specific compound can be associated with the pharmacologic properties of propolis.
Boisard et al. (2015) also reported that different combinations of phenolic compounds involving a complex mechanism of action are essential for biological activity of propolis. For poplar type of propolis, biological activity can be attributed to the synergistic effect of phenolic compounds including cinnamic acid and ester derivatives, including caffeic acid and Caffeic Acid PhenethylEster (CAPE), and flavonoids including pinocembrin, pinobanksin, galangin, chrysin and naringenin (Bankova, 2005; Vardar-Ünlü et al., 2008).
This activity of ethanol fraction of propolis from Nigeria agrees with ethanol extract of propolis from Bulgaria (Paulino et al., 2003), Iran (Parandin and Daroogari, 2019), and China (Sun et al., 2018). It is also in agreement with the result of hydroalcoholic propolis of Brazilian origin (Cavendish et al., 2015), water of Iraqi origin (Abdel Mahdi Kassim Altaee, 2014) and Moroccan origin (Mountassir et al., 2014) which have shown good anti-inflammatory and antinociceptive activities in animal models as reported by Al-Hariri and Abualait (2020).
The findings on analgesic study on propolis are in agreement with studies by Sun et al. (2018) which reported that different fractions from Chinese propolis extracts enriched in polyphenolic constitutions showed central and peripheral antinociceptive effects in animal models. It is also in line with the report of Cavendish et al. (2015) which concluded that the hydroalcoholic extract of Brazillian red propolis exhibited anti-inflammatory and neurogenic pain inhibition without emotional and motor side effects in rodents.
CONCLUSION
The varying polarity of solvents used, has proved that pharmacological activities of sample fractions of propolis is as a result of synergism rather than single fraction action. This can be seen in the good analgesic activity of hexane, a non-polar solvent and ethanol, a highly polar solvent as reported. The results show that this propolis sample is non-toxic at very high doses. It also shows that both non-polar and polar extracts of propolis obtained from Gboko, Benue State have anti-inflammatory and analgesic activities at various dosages.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Abdel Mahdi Kassim Altaee R (2014). Evaluation the Analgesic and Anti-inflammatory Activity of Aqueous Extracts of Iraqi Propolis in Male Albino Mice. Journal of Kerbala University 12(2):1-6. |
|
|
Abozid MM, Ahmed AA (2013). Chemical Composition of Egyptian and Commercial Propolis and its Effects on Liver function and Lipid Profiles in Albino Rats. Journal of Biological, Chemical and Environmental Sciences 8(2):323-340. |
|
|
Adzu B, Amos S, Wambebe C, Gamaniel K (2001). Antinociceptive Activity of Zizyphusspina-christi Root Bark Extract. Fitoterapia 72(4):344-350. |
|
|
Akah P, Nwabie AI (1994). Evaluation of Nigerian traditional medicines: Plants used for rheumatic disorder. Journal of Ethnopharmacology 42(3):179-182. |
|
|
Alencar SM, Oldoni TLC, Castro ML, Cabral ISR, Costa-Neto CM, Cury JA, Rosalen PL, Ikegaki M (2007). Chemical composition and biological activity of a new type of Brazilian propolis: red propolis. Journal of Ethnopharmacology 113(2):278-283. |
|
|
Al-Hariri MT, Abualait TS (2020). Effects of Green Brazilian Propolis Alcohol Extract on Nociceptive Pain Models in Rats. Plants 9(1102):1-9. |
|
|
Amresh G, Reddy GD, Rao Ch V, Singh PN (2007). Evaluation of anti-inflammatory activity of Cissampelos pareira root in rats. Journal of Ethnopharmacology 110(3):526-531. |
|
|
Arvouet-Grand A, Lejeune B, Bastide P, Pourrat A, Privat AM, Legret P (1993). Propolis Extract. I. Acute Toxicity and Determination of Acute Primary Cutaneous Irritation Index. Journal de Pharmacie de Belgique 48(3):165-170. |
|
|
Atta A, Alkofahi A (1998). Anti-nociceptive and Anti-inflammatory Effects of some Jordanian Medicinal Plant Extracts. Journal of Ethnopharmacology 60(2):117-124. |
|
|
Babatunde IR, Abdulbasit A, Oladayo MI, Olasile OI, Olamide FR, Gbolahan BW (2015). Hepatoprotective and Pancreatoprotective Properties of the Ethanolic Extract of Nigerian Propolis. Journal of Intercultural Ethnopharmacology 4(2):102-108. |
|
|
Bankova V (2005). Recent Trends and Important Developments in Propolis Research. Evidence-Based Complementary and Alternative Medicine 2(1):29-32. |
|
|
Bankova V, Castro SLD, Marcucci MC (2000). Propolis: recent advances in chemistry and plant origin. Apidologie 31:3-15. |
|
|
Batista LLV, Campesatto EA, Assis MLB, Barbosa APF, Grillo LAM, Dornelas CB (2012). Comparative study of topical green and red propolis in the repair of wounds induced in rats. Revista do Colegio Brasileiro de Cirurgioes 39(6):515-520. |
|
|
Bispo-Junior W, Miranda EO, Alvino V, Araujo B, Silva DW, Porfirio Z (2012). Antimicrobial activity of fractions of red propolis from Alagoas, Brazil. Semina: Ciências Biológicas e da Saúde Londrina 33(1):3-10. |
|
|
Boisard S, Le Ray AM, Landreau A, Kempf M, Cassisa V, Flurin C, Richomme P (2015). Antifungal and Antibacterial Metabolites from a French Poplar type Propolis. Evidence Based Complementary and Alternative Medicine 10 p. |
|
|
Bonvehi JS, Coll FV, Jorda R (1994). The Composition, Active Components and Bacteriostatic Activity of Propolis in Dietetics. Journal of the American Oil Chemists' Society 71(5):529-532. |
|
|
Brodowska KM (2017). Natural flavonoids: Classification, Potential Role and Application of Flavonoid Analogues. European Journal of Biological Research 7(2):108-123. |
|
|
Bueno-Silva B, Franchin M, Alves CDF, Denny C, Colón DF, Cunha TM, Alencar SM, Napimoga MH, Rosalen PL (2016). Main pathways of action of Brazilian red propolis on the modulation of neutrophils migration in the inflammatory process. Phytomedicine 23(13):1583-1590. |
|
|
Bankova V (2005). Chemical Diversity of Propolis and the Problem of Standardization. Journal of Ethnopharmacology 100(1-2):114-117. |
|
|
Bankova VS, Christov R, Kujumgiev A, Marcucci MC, Popov S (1995). Chemical Composition and Antibactericidal Activity of Brazilian Propolis. Zeitschrift für Naturforschung C 50(3-4):167-172. |
|
|
Castaldo S, Capasso F (2002). Propolis, an old remedy used in modern medicine. Fitoterapia 73(1):1-6. |
|
|
Cavendish RL, de Souza Santos J, Neto RB, Paixão AO, Oliveira JV, de Araujo ED, e Silva AAB, Thomazzi SM, Cardoso JC, Gomes MZ (2015). Antinociceptive and Anti-inflammatory Effects of Brazilian Red Propolis Extract and Formononetin in Rodents. Journal of Ethnopharmacology 173:127-133. |
|
|
Chen C, Shen A (2008). Synergistic Antifungal Activities of Thymol Analogues with Propolis. Natural Product Communications 3(2):279-282. |
|
|
Christov R, Trusheva B, Popova M, Bankov V (2005). Chemical composition of propolis from Canada, its antiradical activity and plant origin. Natural Product Research 19(7):673-678. |
|
|
De Castro SL (2001). Propolis, Biological and Pharmacological activities. Therapeutic uses of this Bee-Product. Annual Review of Biomedical Sciences 3:49-83. |
|
|
Devequi-Nunes D, Machado BAS, Barreto GA, Rebouças Silva J, da Silva DF, da Rocha JLC, Brandão HN, Borges VM, Umsza-Guez MA (2018). Chemical Characterization and Biological Activity of Six different Extracts of Propolis through Conventional Methods and Supercritical Extraction. PLoS ONE 13(12):1-20. |
|
|
Dobrowolski JW, Vohora SB, Sharma K, Shah SA, Naqvi SAH, Dandiya PC (1991). Antibacterial, Antifungal, Antiamoebic, Antiinflammatory and Antipyretic Studies on Propolis Bee Products. Journal of Ethnopharmacology 35(1):77-82. |
|
|
El-Masry TA, Emara AM, El-Shitany NA (2011). Possible Protective Effect of Propolis against Lead-Induced Neurotoxicity in Animal Model. Journal of Evolutionary Biology Research 3(1):4-11. |
|
|
Fazio A, Plastina P, Meijerink J, Witkamp RF, Gabriele B (2013). Comparative Analyses of Seeds of Wild Fruits of Rubus and Sambucus species from Southern Italy: Fatty Acid Composition of the Oil, Total Phenolic Content, Antioxidant and Anti-inflammatory Properties of the Methanolic Extracts. Food Chemistry 140(4):817-824. |
|
|
Fernandez MA, Saenz MT, Garcia MD (1998). Anti-inflammatory Activity in Rats and Mice of Phenolic Acids Isolated from Scrophularia frutescens. Journal of Pharmacy and Pharmacology 50(10):1183-1186. |
|
|
Frozza COS, Garcia CSC, Gambato G, Souza MDO, Salvador M, Moura S, Padilha FF, Seixas FK, Collares T, Borsuk S, Dellagostin OA, Henriques JA, Roesch-Ely M (2013). Chemical characterization, antioxidant and cytotoxic activities of Brazilian red propolis. Food and Chemical Toxicology 52:137-142. |
|
|
Górniak I, Bartoszewski R, Króliczewski J (2019). Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochemistry Review 18(1):241-272. |
|
|
Greenaway W, May J, Scaysbrook T, Whatley FR (1999). Identification by gas chromatography-mass spectrometry of 150 compounds in propolis. Zeitschrift für Naturforschung C 46(1-2):111-121. |
|
|
Havsteen B (1983). Flavonoids, a Class of Natural Products of High Pharmacological Potency. Biochemical Pharmacology 32(7):1141-1148. |
|
|
Ichikawa H, Satoh K, Tobe T, Yasuda I, Ushio F, Matsumoto K, Endo K, Ookubo C (2002). Free Radical Scavenging Activity of Propolis. Redox Report 7(5):347-350. |
|
|
Inui S, Hatano A, Yoshino M, Hosoya T, Shimamura Y, Masuda S, Ahn MR, Tazawa S, Araki Y, Kumazawa S (2014). Identification of the Phenolic Compounds Contributing to Antibacterial Activity in Ethanol Extracts of Brazilian Red Propolis. Natural Product Research 28(16):1293-1296. |
|
|
Ipav SS, Moronkola DO, Aiyelaagbe OO (2018). Analgesic Activities of Leaf, Stem and Root Essential Oils of Corchorus olitorius L. (Tiliaceae) from Nigeria. Journal of Applied Biosciences 123:12388-12395. |
|
|
Khalil ML (2006). Biological activity of bee propolis in health and disease. Asian Pacific Journal of Cancer Prevention 7(1):22-31. |
|
|
Li F, Awale S, Tezuka Y, Kadota S (2008). Cytotoxic constituents from Brazilian red propolis and their Structure-Activity Relationship. Bioorganic & Medicinal Chemistry 16(10):5434-5440. |
|
|
Li YC, Xian YF, Ip SP, Su ZR, Su JY, He JJ, Xie QF, Lai XP, Lin ZX (2011). Anti-inflammatory Activity of Patchouli Alcohol Isolated from Pogostemonis herba in Animal Models. Fitoterapia 82(8):1295-1301. |
|
|
Marcucci MC, DeCamargo FA, Lopes CMA (1996). Identification of amino acids in Brazilian propolis. Zeitschrift für Naturforschung C 51(1-2):11-14. |
|
|
Mirazi N, Hosseini A (2013). Evaluation of Antinociceptive Activity of Berberis Vulgaris L. Fruit's Hydroethanolic Extract in Male Mice. Iranian Journal of Pharmaceutical Sciences 9(4):15-22. |
|
|
Mountassir M, Chaib S, Selami Y, Khalki H, Ouachrif A, Moubtakir S, Aboufatima R, Zyad A, Benharref A, Chait A (2014). Antinociceptive Activity and Acute Toxicity of Moroccan Black Propolis. International Journal of Engineering Research and Technology 3(2):2393-2397. |
|
|
Murugan R, Parimelazhagan T (2013). Study of Anti-nociceptive, Anti-inflammatory Properties and Phytochemical Profiles of Osbeckia parvifolia Arn. (Melastomataceae). Industrial Crops and Products 51:360-369. |
|
|
Nande WU, Igoli JO (2017). Isolation and Characterisation of bioactive compounds from plant materials: The Nigerian situation. Nigerian Journal of Pure and Applied Sciences 9(1):132-143. |
|
|
Parandin R, DaroogariS (2019). Anti-Inflammatory and Antinociceptive Activities of the Ethanolic Extract of Propolis in Male Mice and Rats. Zahedan Journal of Research in Medical Sciences 21(2):1-7. |
|
|
Paulino N, Dantas AP, Bankova V, Longhi DT, Solange AS, de Castro L, Calixto JB (2003). Bulgarian Propolis Induces Analgesic and Anti-inflammatory Effects in Mice and Inhibits In Vitro Contraction of Airway Smooth Muscle. Journal of Pharmacological Sciences 93(3):307-313. |
|
|
Pimenta HC, Violante IM, Musis CR, Borges ÁH, Aranha AM (2015). In vitro Effectiveness of Brazilian Brown Propolis against Enterococcus faecalis. Brazilian Oral Research 29(1):1-6. |
|
|
Razmi A, Zarghi A, Arfaee S, Naderi N, Faizi M (2013). Evaluation of Anti-nociceptive and Anti-inflammatory Activities of Novel Chalcone Derivatives. Iranian Journal of Pharmaceutical Research 12:153-159. |
|
|
Ristivojevi? P, Trifkovi?b J, Andri? F, Milojkovi?-Opsenicab D (2015). Poplar-type Propolis: Chemical Composition, Botanical Origin and Biological Activity. Natural Product Communications 10(11):1869-1876. |
|
|
Santos FA, Rao VSN, Silveira ER (1998). Investigations on the antinociceptive effect of Psidium guajava leaf essential oil and its major constituents. Phytotherapy Research 12(1):24-27. |
|
|
Seidel V, Peyfoon E, Watson DG, Fearnley J (2008). Comparative Study of the Antibacterial Activity of Propolis from Different Geographical and Climatic Zones. Phytotherapy Research 22(9):1256-1263. |
|
|
Sforcin JM (2016). Biological Properties and Therapeutic Applications of Propolis. Phytotherapy Research 30(6):894-905. |
|
|
Sun L, Liao L, Wang B (2018). Potential Antinociceptive Effects of Chinese Propolis and Identification on Its Active Compounds. Journal of Immunology Research Article ID 5429543 6 p. |
|
|
Teixeira EW, Message D, Negri G, Salatino A, Stringheta PC (2008). Seasonal variation, chemical composition and antioxidant activity of Brazilian propolis samples. Evidence Based Complementary and Alternative Medicine 7(3):307-315. |
|
|
Tor-Anyiin TA, Igoli JO, Anyam JV, Anyam JN (2015). Isolation and antimicrobial activity of sarracenin from root bark of Strychnos spinosa. Journal of Chemical Society of Nigeria 40(1):71-75. |
|
|
Uwaya DO, Okakwu R, Omozuwa OP (2020). In-Vivo and In-Vitro Anti-Inflammatory Activities of the Aqueous Extract of Di Herbal Formulation (Euphorbia hirta and lactuca virosa). Journal of Applied Sciences and Environmental Management 24(11):1979-1985 |
|
|
Vardar-Ünlü G, Silici S, Ünlü M (2008). Composition and In vitro Antimicrobial Activity of Populus Buds and Poplar-type Propolis. World Journal of Microbiology and Biotechnology 24(7):1011-1017. |
|
|
Wang Y, Chen P, Tang C, Wang Y, Li Y, Zhang H (2014). Antinociceptive and Anti-inflammatory Activities of Extract and Two Isolated Flavonoids of Carthamus tinctorius L. Journal of Ethnopharmacology 151(2):944-950. |
|
|
Wieczynska A, Wezgowiec J, Wieckiewicz W, Czarny A, Kulbacka J, Nowakowska D, Gancarz R, Wilk KA (2017). Antimicrobial Activity, Cytotoxicity and Total Phenolic Content of Different Extracts of Propolis from the West Pomeranian Region in Poland. Acta Poloniae Pharmaceutica 74(2):715-722. |
|
|
Xu Y, Lin D, Yu X, Xie X, Wang L, Lian L, Fei N, Chen J, Zhu N, Wang G, Huang X, Pan J (2016). The Antinociceptive Effects of Ferulic Acid on Neuropathic Pain: Involvement of Descending Monoaminergic System and Opioid Receptors. Oncotarget 7(15):20455-20468. |
|
|
Xu Q, Wang Y, Guo S, Shen Z,Wang Y, Yang L (2014). Anti-inflammatory and Analgesic Activity of Aqueous Extract of Flospopuli. Journal of Ethnopharmacology 152(3):540-545. |
|
|
Yamanishi R, Yoshigai E, Okuyama T, Mori M, Murase H, Machida T, Okumura T, Nishizawa M (2014). The Antiinflammatory Effects of Flavanol-Rich Lychee Fruit Extract in Rat Hepatocytes. PLoS One 9(4):e93818. |
|
|
Yonathan M, Asres K, Assefa A, Bucar F (2006). In vivo Anti-inflammatory and Anti nociceptive Activities of Cheilanthes farinosa. Journal of Ethnopharmacology 108(3):462-470. |
|
|
Zhang Y, Shu Z, Yin L, Ma L, Wang X, Fu X (2015). Anti-inflammatory and Antinociceptive Activities of Non-Alkaloids Fractions from Aconitum flavum in vivo. Revista Brasileira de Farmacognosia 25(1):47-52. |
|
|
Zhao Y, Liu J, Liu C, Zeng X, Li X, Zhao J (2016). Anti-Inflammatory Effects of p-coumaric Acid in LPS Stimulated RAW264.7 Cells: Involvement of NF-kB and MAPKs Pathways. Medicinal Chemistry 6(5):327-330. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0