The present study was designed to investigate the antioxidant and anticancer properties of Ficus carica (FC). The dried coarse powder of F. carica fruits was exhaustively extracted with ethanol and the resulting crude ethanolic extract (FCE) was assayed for antioxidant and anticancer activities, since both are inter-related. The antioxidant activity of FCE was determined by reductive potential in reducing ferric ions and anticancer activity was determined using breast cancer cell lines (MCF7). The extract showed strong antioxidant and anti-cancer activities, which suggest the use of the plant for therapeutic purposes, supporting the traditional claims.
One of the largest causes of mortality worldwide is cancer. So far, abnormalities in about 350 genes have been demonstrated in human cancers (Futreal et al., 2004; Broadhead et al., 2010) and epidemiologically, cancer is responsible for one in eight of worldwide deaths (Wolf and Davidovici, 2010). Increasing interest and research on herbal medicine have revealed its impor-tance in treating many diseases including cancer. Epidemiologic studies have suggested that some antioxidant agents as well dietary constituents with antioxidant properties may be acting as naturally occur-ring cancer prevention agents. Therefore, there is an urgent need to develop alternative therapeutic measures against this deadly disease. Many components from dietary or medicinal plants have been identified that they possess substantial chemopreventive properties. India has unique plant varieties yet to be studied for anticancer components. Breast cancer is the second most common cancer amongst women, comprising 21.4% of female cancers. Antioxidants also have known to play a vital role in preventing cancers. Several phytocompounds which possess antioxidant potential are known to prevent cancers and the anticancer effects may be associated with antioxidant properties due to its polyphenolic components (Gheldof et al., 2003; Liu et al., 2000; Laandrault et al., 2001; Siddhuraju and Becker, 2003; Zuo et al., 2002). Among the several plants reported to possess medicinal properties, Ficus constituted one of the largest genera of medicinal plants with about 750 species of woody plants, trees, and shrubs primarily occurring in subtropical and tropical regions throughout the world. The genus is remarkable for the large variation in the habits of its species. In India, the most important species of Ficus are Ficus bengalensis, Ficus carica,
Ficus racemosa and Ficus elastica. F. carica is commonly referred as “fig". Various parts of the plant like bark, leaves, tender shoots, fruits, seeds, and latex are medicinally important. The fig is a very nourishing food and used in industrial products. It is rich in vitamins, mineral elements, water, and fats. Figs are one of the highest plant sources of calcium and fiber.
According to USDA data for the Mission variety, dried figs are richest in fiber, copper, manganese, magnesium, potassium, calcium and vitamin K relative to human needs. They have smaller amounts of many other nutrients. Figs have a laxative effect and contain many antioxidants inhibiting the oxidative mechanisms that may lead to degenerative illnesses (du Toit, 2001). They are good source of flavonoids and polyphenols and some bioactive compounds such as arabinose, β-amyrins, β- carotines, glycosides, β-setosterols and xanthotoxol. The dried figs produced a significant increase in plasma antioxidant capacity and also used in various disorders such as gastrointestinal respiratory, inflammatory, cardio-vascular disorders, ulcerative diseases, and cancers. In traditional medicine, the roots are used in treatment of leucoderma and ringworms and its fruits which are sweet, have antipyretic, purgative, aphrodisiac properties and have shown to be useful in inflammations and paralysis. F. carica has been reported to possess antioxidant activity by previous study (Solomon et al., 2006). The plant has been reported to possess antiviral, antibac-terial, hypoglycemic, hypo triglyceridaemic and anthelmintic effects. There are no reports on the F. carica crude ethanolic extract (FCE) possessing both antioxidant and anticancer activity. Hence, this study was aimed to investigate the antioxidant and anticancer activity of F. carica fruits. The present review correlates antioxidant activity of Ficus species with its anticancer activity.
Collection of plant materials
F. carica fruits were purchased from local supermarket at Tiruchirappalli District, Tamil Nadu, India during the month of March and authenticated by the botany department of our college and the voucher specimen was deposited.
Preparation of plant extract
The fruits chosen for the study were been washed, macerated and lyophilised. The collected fruits were chopped into small pieces, sun dried for about five days and grinder into coarse powder with a mechanical grinder and stored in an air tight container. Then 25 g of powder was taken mixed with 100 ml of ethanol for five days at room temperature 25 ± 2°C with occasional stirring. After 5 days the ethanol extract was filtered with Whatman No. 1 filter paper. The extract was concentrated under reduced pressure below 50°C through rotary vaccum evaporator. The concentrated extract was collected in a Petri dish and allowed to dry for complete evaporation of ethanol. The whole process was repeated three times and finally, collected the blackish green color, concentrated plant extract. It was stored at 4°C for future use. About 500 g of fruits yielded 37 g powder. The procedure was repeated to collect the needed quantity.
Cell line and culture
Breast cancer (MCF-7) cell line was obtained from NCCS, Pune, India. The cells were maintained in minimal essential media supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml) in a humidified atmosphere of 50 μg/ml CO2 at 37°C.
Reagents
MEM, Trypsin, methylthiazolyl diphenyl- tetrazolium bromide (MTT), and dimethyl sulfoxide (DMSO) were needed for the study. All the chemicals and reagents were obtained from Sigma Aldrich Mumbai, India.
In vitro assay for anticancer activity (MTT assay)
The anticancer activity of FCE on MCF-7 was determined by the MTT assay (Mosmann, 1983). Cells (1 × 105/well) were plated in 1 ml of medium/well in 24-well plates (Costar Corning, Rochester, NY). After 48 h of incubation, the cells reached the confluence; then the cells were incubated in the presence of various concentrations of the samples in 0.1% DMSO for 24, 48 and 72 h at 37°C. After removal of the sample solution and washing with phosphate-buffered saline (pH 7.4), 200 µl/well (5 mg/ml) of 0.5% MTT solution was added. After 4 h incubation, 0.04 M HCl/ isopropanol were added. Viable cells were determined by the absorbance at 570 nm. Measurements were performed and the concentration required for a 50% inhibition of viability (IC50) was determined. The absorbance at 570 nm was measured with a UV- spectrophotometer using wells without sample containing cells as blanks. The effect of the samples on the proliferation of MCF-7 was expressed as the % cell viability, using the following formula:
% cell viability = (O.D at 570 of treated cells / O.D at 570 of control cells) × 100%.
Reducing power assay
Principle
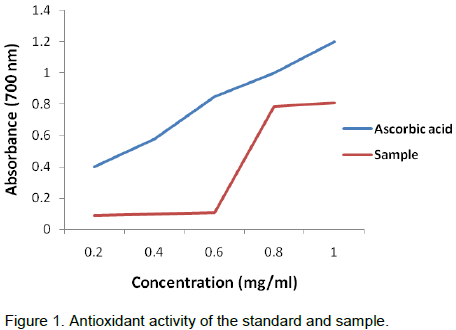
The reducing power of ethanol extract of Ficus fruits was determined by the reducing antioxidant power method, and was conducted according to Pellegrini et al. (2003). Substances, which have reduction potential, react with potassium ferricyanide (Fe3+) to form potassium ferrocyanide (Fe2+), which then reacts with ferric chloride to form ferric ferrous complex that has an absorption maximum at 700 nm.
Chemicals required
Potassium ferricyanide (1% w/v), phosphate buffer (0.2 M, pH 6.6), trichloro acetic acid (10%), ferric chloride (0.1%) and ascorbic acid (1%).
Phosphate buffer preparation
Dibasic sodium phosphate (18.75 ml of 0.2 M) is mixed with 31.25 ml monobasic sodium phosphate and diluted to 100 ml with water.
Procedure
Various concentrations of the plant extracts in 1.0 ml of deionized water were mixed with phosphate buffer (2.5 ml) and potassium ferricyanide (2.5 ml) and incubated at 500°C for 20 min. Aliquots of trichloroacetic acid (2.5 ml) were added to the mixture, which was then centrifuged at 3000 rpm for 10 min whenever necessary. The upper layer of solution (2.5 ml) was mixed with distilled water (2.5 ml) and a freshly prepared ferric chloride solution (0.5 ml). The absorbance was measured at 700 nm. A blank was prepared without adding extract. Ascorbic acid at various concentrations was used as standard; increased absorbance of the reaction mixture indicates increase in reducing power.
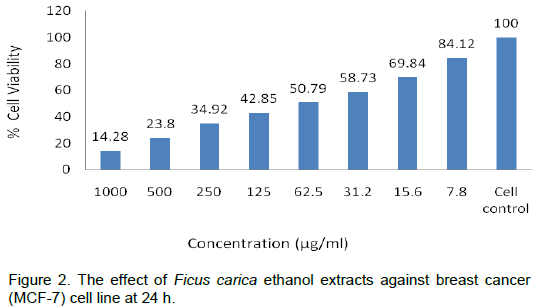
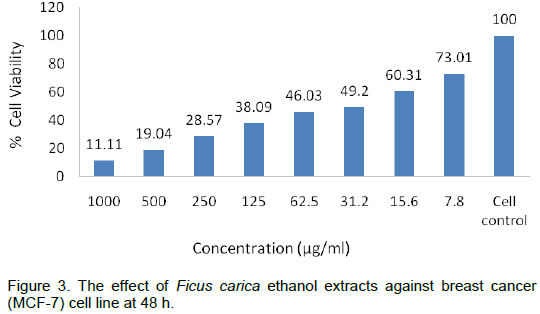
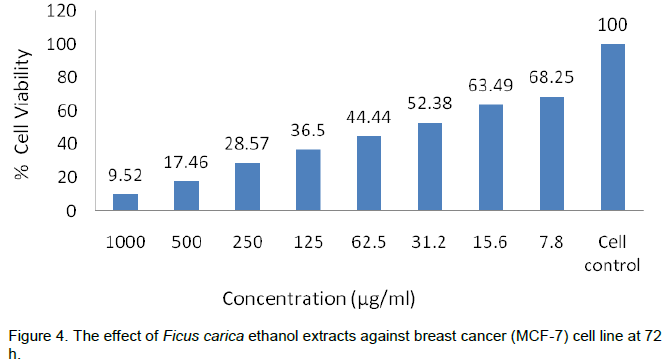
The anticancer activities of F. carica ethanol extracts at 24, 48 and 72 h have been shown in Tables 2 to 5 and Plates 1 to 3. The results show a dose and time depen-dent decrease in viability. The percentage of cell death at 1000 µg/ml varied in
time and was 85.5% at 24 h, 89% at 48 h and 90.5% at 72 h, while at dose of 500 µg/ml, the response was also time dependent as the 76% inhibition at 24 h increased to 80.5 and 82.5% at 48 and 72 h, respectively (Figures 2 to 4). This was also supported by the antioxidant activity as depicted in Table 1 and Figure 1. Similar findings were already reported by Jeune et al. (2005). The ability of plant extracts to significantly decrease the cell viability in a time and dose dependent manner had also been observed by other researchers (Svejda et al., 2010). There has been growing interest in the beneficial health effects of consuming fruits and vegetables. Mainly, the presence of phenolic antioxidants is believed to have
the protective mechanisms (Sun, 1990). Phenols have always known to play a vital role in alleviating cancers and also possess antioxidant activity (Rice-Evans et al., 1996; Son and Lewis, 2002).
In the present study, the ethanol extracts of F. carica exhibited reducing activity and anti-cancer activity. The results indicate that the extent of antioxidant activity of the extract is in accordance with the anti-cancer activity. The most common cause of hereditary breast cancer is an inherited mutation in the BRCA1 and BRCA2 genes. In normal cells, these genes help prevent cancer by making proteins that keep the cells from growing abnormally. Although, in some families with BRCA1 mutations, the lifetime risk of breast cancer is as high as 80%, on average, this risk seems to be in the range of 55 to 65%. For BRCA2 mutations, the risk is lower, approximately 45%. Breast cancers linked to these mutations occur more often in younger women and more often affect both breasts than cancers not linked to these
mutations. Women with these inherited mutations also have an increased risk for developing other cancers, particularly ovarian cancer. It has been reported that ethanolic extracts are normally used for anticancer screening because it is believed that the polar compounds were mostly claimed for anticancer properties. As mentioned formerly, the ethanolic extracts of FC showed significant cytotoxic activities towards MCF-7 cells. The anti-cancer activity was also supported by the anti-oxidant activity displayed by FCE (Chiang et al., 2005).
The present results, together with previous studies, suggest that Ficus extract has great potential as an anticarcinogenic and antioxidant agent. In general, the beneficial effect of plant products such as Ficus may be attributable to one or more phytochemicals including antioxidants, phenolic compounds and other substances. Understanding the modes of action of these compounds should provide useful information for their possible applications in cancer prevention and perhaps in cancer therapy (Taraphdar et al., 2001; David, 2004). Further work to identify the active principle and its role in signaling the key apoptotic enzymes is under progress.
This study has provided insight towards the possibility of using plants effectively as a possible alternative treatment for cancer treatments, supporting the traditional use of this plant. Further fractionation and spectral analysis are under progress to identify an effective and safer drug for anti-oxidant and anti-cancer activities. Experimental results reveal that the plant extract possesses significant anticancer activity which may be due to its cytotoxicity and antioxidant properties. Further research is going on to find out the active principle(s) of FCE for its anti-cancer activity.
The authors have not declared any conflict of interests.