ABSTRACT
This study investigated fungi and total aflatoxins quality of ogiri [fermented melon seed (Citrullus vulgaris] and fermented African oil bean seed (Pentaclethra macrophylla) consumed as soup condiment in Abakaliki, Ebonyi State, Nigeria. The ogiri samples were purchased from two major markets in Abakaliki metropolis [meat market (OM and UM) and rice mill market (OR and UR)]. The samples were screened for their pH values, total fungi count and total aflatoxins content. A total of four samples were analzed for six weeks. The total aflatoxins were analyzed using highly sensitive competitive enzyme link immunosorbent assay (ELISA) reader. The ogiri samples were also analyzed for total fungal count using a digital colony counting machine (CCM China). The result showed that all the ogiri samples analyzed were heavily contaminated by the fungal cells. The total mean fungal count for the ogiri processed from melon seed were 2.2 × 107 and 2.2 × 107 cfu/g for OM and OR, respectively while the ogiri processed from African oil bean were 1.4 × 107 and 1.6 × 107 cfu/g for UM and UR samples, respectively. The ogiri samples also contained unacceptable levels of aflatoxins as the average total aflatoxins for all the samples were above the minimum acceptable limits (10 ppm), according to the National Agency for Food and Drug Administration and Control (NAFDAC) as reported by the Food and Drug Administration (FDA) of USA. The research also revealed that ogiri samples have high moisture content. The study recommends that more improved process line be put in place to ensure that all ogiri sold in Abakaliki are produced using Standard Operating Procedure (SOP).
Key words: Ogiri, aflatoxins, fungi, African oil bean, melon seed, enzyme link immunosorbent assay (ELISA).
Food condiments (seasoning/spices) are substances added to food to impact a particular flavor or to generally improve the sensory/chemical quality of the food/food products (Odibo et al., 1990; Njoku et al., 1990). Fermented food seasonings or local seasoning are those seasonings which undergo traditional foods processing method, that involves biochemical changes brought about by microbes inherent in the food or derived from starter culture and their enzymes. These local seasonings can also add extra nutrients such as vitamins B to the food. The use of fermented proteins rich seeds as seasonings is wild spread in Africa and Asia (Sanni, 1993).
The fermented protein rich seeds in addition to the flavor also act as protein supplements and functional ingredients in the processed foods (Achi, 2005). These seasonings are being increasingly marketed throughout the world today. Ogiri is a Nigerian fermented condiment produced from various substances, and which when added to soup or yam porridge enhances the flavor. Several research has been carried out on the production of ogiri from the fermentation of African oil bean seed (Pentaclethra macrophylla) creeping melon (Colocynthis vulgaris) (Odunfa, 1985; Jideani and Okeke, 1991; David and Aderibigbe, 2010), Citrullus lanatus (David and Aderibigbe, 2010), seeds of castor oil (Ricinus comminus) (Odunfa, 1985; Jideani and Okeke, 1991), watermelon seed (Citrullus vulgaris) (Odunfa, 1985) and fluted pumpkin (Telfairia occidentalis Hook) (Odibo and Umeh, 1989). The choice of substrate for this food condiment, which is popular among many people in Nigeria, depends on the locality (Odibo et al., 1990).
Ogiri ugba is a value added semi-solid fermented product of African oil bean seed (P. macrophylla), prepared by the igbos in eastern Nigerian. Fermentation detoxifies the African oil bean seed with subsequent increase in nutrient availability and digestibility. Ogiri Ugba is widely used as a soup condiment (flavor enhancing condiment) (Mbata and Orji, 2008) with its production locally done through a mixed wide bacteria fermentation of sliced, boiled and soaked African oil bean seeds. Unprocessed oil bean seeds are bitter and possess anti-nutritional factors among which are pancine, cyanide, oxalates, saponin, phytates and tannins (Achinewhu, 1983; Enuijiugha and Akanbi, 2005; Onwuliri et al., 2004). The microbial population of Ogiri ugba is introduced through the air, water, utensil, banana leaves or the handler and no starter culture is used in the traditional method. Microorganisms often involved are predominantly Bacillus spp., Micrococcus and Lactobacillus spp., Pseudomonas spp., Staphyloccoccus spp., Enterobacter spp., Leuconostoc spp, Corynebacterium and Alkaligenes spp (Enuijiugha, 2009; Isu and Njoku, 1997; Isu and Ofuya, 2000; Njoku et al., 1990; Obeta, 1983; Sanni et al., 2000).
Melon seed (C. vulgaris) has high protein and low carbohydrate content and belong to the family Cucurbitaceae (Alfred, 1986). Ogiri-egusi is characterized by very strong pungent odour which turns into a pleasant aroma upon cooking as soup condiment. Among the consumers, their preferences for either ogiri-ugba or Ogiri egusi vary among localities.
Production of these condiments (ogiri-ugba and Ogiri-egusi) by local technology creates room for contamination by diverse microorganisms. The fermentation environment also supports and encourages the growth and multiplication of potentially toxigenic fungi thus leading to the production of diverse forms of mycotoxins. Aflatoxins are secondary metabolite produced by fungi with toxicological properties, that induces a variety of health challenges when foods contaminated with these compounds are ingested. Aflatoxins are stable under most processing conditions and therefore persist to the final products (Oranusi et al., 2013). In this work, the microbes and total aflatoxins analysis of ogiri-ugba and Ogiri-egusi produced and consumed in Abakaliki metropolis was carried out. The findings of this study will serve the purpose of alerting consumers on the possible danger of consuming poorly processed Ogiri (ogiri-ugba and Ogiri-egusi) on sale in selected markets within Abakaliki Ebonyi State, Nigeria.
Samples collection
The Ogiri-egusi [processed from melon seed (C. colocynthis)] and ogiri-ugba [processed from African Oil bean seed (P. macrophylla)] used in this research were purchased from meat market and rice mill market, Abakaliki, Ebonyi State. Ogiri-egusi purchased from meat market was designated as OM while that purchased from rice mill market was designated as OR. Also, Ogiri-ugba purchased from meat market was designated as UM whereas that purchased from rice mill market was designated as UR.
Determination of moisture content of the Ogiri samples
The moisture content was determined by the gravimetric method. A measured weight of each sample (5 g) was weighed into a cleaned, dried Petri dish. The dish and samples were dried in an oven at 105°C for 3 h at first instance. It was then cooled in a desiccator and reweighed.
The weight was recorded while the samples were returned to the oven for further drying. The drying, cooling and weighing continued repeatedly until a constant weight was obtained. By the difference, the weight of the moisture loss was determined and expressed as a percentage. It was calculated as shown below:
Where, W1 = weight of the empty Petri dish; W2 = weight of the dish and sample before drying; W3 = weight of the dish and sample after drying to a constant weight
pH determination
The pH of the samples was determined using highly sensitive digital pH meter (Montini 095, Romania). Five grams (5 g) of each sample was weighed and transferred to a clean beaker and 50 ml of distilled water was added to form a slurry.
A standard buffer solution (pH 6.0) was prepared and was used to standardize the pH meter. The electrode of the digital pH meter was dipped in the slurry at a temperature of about 29°C. The pH readings were recorded.
Total viable fungal count
Ten-fold serial dilution and pour plate method were used for the fungal count. The medium used (Saboraud Dextrose Agar) were prepared according to manufacturer‘s instructions (BioTech India) and autoclave for 15 min at 121°C and 15 psi. The prepared medium was allowed to cool to about 40°C in a water bath and was then poured into sterile Petri-dishes containing 1 ml aliquots of the appropriate dilutions (normal saline as diluents) prepared from the samples.
The samples solutions were prepared by adding 1 g of the sample into 10 ml of normal saline. The plates were incubated for 3 days at room temperature and colonies formed were counted using digital colony counter and expressed in colony forming unit per gram CFU/g.
Total aflatoxins analysis
Determination of total aflatoxins on the ogiri samples was done by the use of highly sensitive competitive Enzyme linked immunosorbent assay (ELISA) reader. Extraction of the aflatoxin was done with Tween-ethanol. 25 mm of Tween-ethanol was added to 5 g of the sample and mixed properly. The sample solution was then centrifuged at 250 rpm for 3 min, and filtered using Watman1 filter paper.
Aflatoxin conjugate (200 mL) was dropped in a clean mixing wall and 100 µl of the sample analyte was added. The mixture of the aflatoxin conjugate and the sample was then transferred into antibody incubated micro-walls and incubated under dark cover at room temperature 26 ± 2°C for 15 min. This process allowed the antibody/antigen reaction to take place. After incubation, the solution was then washed off 5 times using deionized water then, 100 µl of the substrate was added and allowed to stand for 5 min. Finally, a stop solution was added and the result was read by ELISA reader.
Ogiri are prone to fungal contamination and spoilage due to poor local processing and packaging method. This study revealed that, the severity of the processing contamination and spoilage varied among the different ogiri samples, are reflected in different levels of hygienic processes that were used to produce ogiri. Thus, the total fungal populations and the concentrations of total aflatoxins varied among the ogiri samples.
pH values
The pH analysis showed that the different ogiri samples have different pH that ranges from 6.2 to 7.2 and 6.5 to 8.5 for ogiri-egusi and Ogiri ugba, respectively (Figure 1). pH is an important factor that influences the microbial content of any food material. The variation in the pH values of the ogiri samples could be as a result of the varied fermentation time. According to Achi (2005), the pH of traditionally fermented protein condiments is significantly affected by the fermentation time. The variety of the melon seed and the African oil bean seed could also be a factor, that might have influenced the pH as genetic composition of the seeds, which have been found to determine the chemical composition of the seeds and thus their pH (Achinewu, 1987).
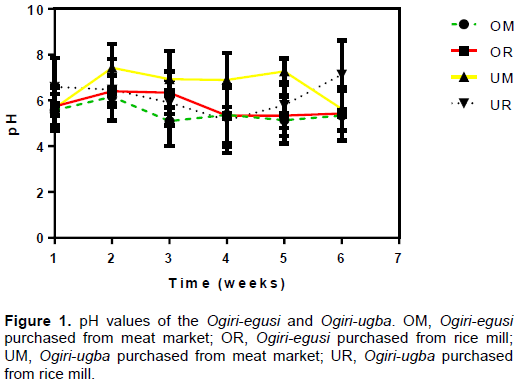
The high pH of fermented legumes (melon seed) and African oil bean seed compared to other materials (cereals) under similar conditions have been attributed to high protein content of the seeds (Zamora and Fields, 1979). According to Achinewhu (1986), unsaturated fatty acids are increased with the hydrolysis of protein in amino acids and peptides. Ammonia is released to the proteolytic activity taking place during fermentation, which therefore raises the pH of the final products and giving the food a strong ammonical odour and flavor (Wang, 1996). This we referred to as “Alkaline fermentation” which aids in prolonging the shelf life of such product.
The result of this research is however, in line with the findings of other researchers as observed by David and Aderibigbe (2010) for ogiri from different melon seeds. Odunfa (1981) in his work described the fermentation process as essentially putrefactive, noting that the increase in pH was probably due to the formation of ammonia by the deaminase enzymes of Bacillus and Proteus spp. that often involve in the fermentation process.
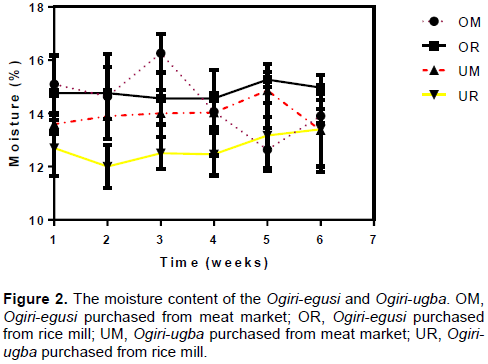
Moisture content
The moisture content of the ogiri samples was found to be between 12.5 to 15.1% and 11.8 to 13.9% for ogiri-egusi and ogiri-ugba, respectively (Figure 2). This suggests that the ogiri samples were not sufficiently dried after processing.
Moisture content is a key factor that affects the microbial quality of any processed food/food products. Food or food products with high moisture content tend to have higher microbial content and spoil faster than those with lower moisture contents. This result is similar to the finding of David and Aderibigbe (2010), who reported that high moisture content of melon seed significantly affects the overall chemical composition of the ogiri made from the melon seed. The high moisture content also affects the microbial content of ogiri made from melon seed according to the research done by Odibo and Umeh (1989) and Nwagu et al. (2010).
Total fungi count
The finding of this research shows that the different ogiri samples analyzed were heavily contaminated with fungi (Table 1). The ogiri ugba has the lowest average fungal populations (1.2 x 107 - 1.9 x 108) while the ogiri egusi had the highest fungal cells (2.3 x 107 - 2.5 x 108) during the study period. There was a significant difference among the fungal populations of the ogiri samples.
The level of fungal load in the ogiri sample revealed the extent of fermentation as well as the storage stability of ogiri samples. Ogiri that have had longer fermentation time tend to have higher fungal populations and also tend to spoil faster than those with lower fungal populations. This is because with higher fungal population, the rate of metabolic activities of the fungal cells on the ogiri becomes faster resulting in production in high proportion of certain undesirable metabolites which subsequently lead to off-flavors and general change in the chemical composition of the flour. This high fungal population seen in these ogiri samples could be due to poor processing resulting in cross-contaminations of the ogiri from both the traditional processing equipments and the personnel.
The presence of high fungal cells in all the samples could also be attributed to the normal flora of the seeds. Normal microbial flora have been reported to withstand processing procedures and conditions and could be found in final products. Fungi are common environmental contaminants and are known to produce spores that survive unfavourable environmental conditions; this could explain their presence in this food condiments (Oranusi et al., 2013). The levels of contaminants could be associated to contamination from the environment, the food vendors and personnel involved in the production process (De Roever, 1998; Beuchat, 2002). It has been reported that, the microbial contamination of a product is dependent on the environment it passed through and to sanitary quality of the processing water, transportation, storage and processing of the produce (EC-SCF, 2002; Oranusi and Braide, 2012; Buck et al., 2003; WHO, 2008).
The result of this research is similar to the findings of other researchers in which they reported that, Ogiri condiments are fermented products often contaminated by diverse species of fungi due to poor local technology (Odunfa, 1981; Adenike and Kehinde, 2008; Nwagu et al., 2010; Oranusi et al., 2013).
Total aflatoxin
The finding of this research showed that the ogiri samples were contaminated with different concentrations of aflatoxins ranging from 10.0 to 15.2 ppb and 11.1 to 21.1 ppb for ogiri-egusi and ogiri-ugba, respectively (Table 2). The level of aflatoxins is one of the key safety and quality indicator parameter of any processed food/food products. Aflatoxin has been reported to be produced under conducive environmental condition of high water activity and high pH (Zuber et al., 1993). Aflatoxins are potent natural carcinogenic substance linked to higher prevalence of hepatocellular cancer in Africa (Strosnider et al., 2006). It has also been revealed that the highest risk occurs with hepatitis B and C carriers in developing cancer, when exposed to aflatoxin (Williams et al., 2003). Other studies also linked aflatoxin to immunosuppression and stunted growth in children (Turner et al., 2003; Wu and Khlangwiset, 2010).
Liver is the principal organ affected by aflatoxin. In the liver, aflatoxin may be transformed by certain P450 enzyme to its DNA reactive form aflatoxin-8,9-epoxide. These molecules may bind to liver proteins and lead to their failure, potentially resulting to active aflatoxicosis. Most food products, ogiri condiments inclusive with total aflatoxins level beyond 10 ppm are designated as unfit for human consumption according to National Agency for Food and Drug Administration and Control (NAFDAC). This levels of total aflatoxin in the food commodities were generally above the maximum allowable limits (10 ppb for food meant for domestic consumption) specified by the European Commission (AESAN, 2011), which is also currently being used NAFDAC in Nigeria. This is because consumption of foods with such high levels of aflatoxins contaminations has been linked with both acute and chronic heart diseases including cancer (Azi et al., 2016).
It has been established that over 5 billion people, mostly in developing countries, are at risk of chronic exposure to aflatoxins from contaminated foods; food condiments inclusive (Shuaib et al., 2010). This high concentration of aflatoxins revealed in this study could be as results of high fungal contamination of the ogiri condiments. The finding of this research is similar to the investigative report of Food and Agriculture Organization (FAO) and Food and Drug Administration (FDA) risk assessment report on aflatoxins in foods, in which they discovered different concentrations of aflatoxins in the different foods assessed, including food condiments.
The finding of this research showed that processed ogiri which is consumed in Abakaliki are heavily contaminated with molds, with potential for aflatoxins production. The aflatoxins analysis also revealed unacceptable levels of aflatoxins in the ogiri samples. It is therefore recommended that urgent review of the entire process line for ogiri sold in Abakaliki metropolis, is carried out to ensure that all ogiri sold in Abakaliki are produced following standard operating procedures (SOP).
Also, the processed ogiri should be stored at dry and cool environment (temperature preferably below 20°C and relative humidity below 80%), to reduce the chance of fungal contamination and proliferation during storage. There is also an urgent need to educate producers of food condiments, food vendors and consumers on the dangers poor food handling and storage and the need to apply Good Manufacturing Practices (GMP) and Hazard Analysis Critical Control Point (HACCP) in processing of ogiri in Abakaliki metropolis.
The authors have not declared any conflict of interests.
REFERENCES
|
Achi OK (2005). Traditional Fermented Protein Condiments in Nigeria. Afr. J. Biotechnol. 4(3):1612-1621.
|
|
|
|
Achinewhu SC (1983). Protein of the African Oil bean (Pentaclethra macrophylla). J. Food Sci. 48:1374-1375.
Crossref
|
|
|
|
|
Achinewhu SC (1986). The effect of fermentation on carbohydrate and fatty acid composition of African oil bean seed (Pentaclethra macrophylla). Food Chem. 19: 105-116.
Crossref
|
|
|
|
|
Achinewu SC (1987). Carbohydrate and Fatty Acid Composition of Fermented Melon Seeds (Citrullus vulgaris). J. Food Sci. Technol. 24:16-19.
|
|
|
|
|
Adenike AO, Kehinde OO (2008). Microbial load and Incidence of Food-Borne Indicator bacteria in most Popular Indigenous Fermented food Condiments from middle-belt and Southwest Nigerian. Afr. J. Microbiol. Res. 2:323-339.
|
|
|
|
|
AESAN (2011). Report of the Scientific Committee of the Spanish Agency for Food Safety and Nutrition (AESAN) in relation to the effect on the Spanish population of the derogation of national regulation on maximum allowed limits for aflatoxins B1, B2, G1 & G2 in food. pp. 1-16.
|
|
|
|
|
Alfred BG (1986). Tropical colour encyclopedia of exotic plants and trees, 3rd Edition. New York: Acad Press London, 472p.
|
|
|
|
|
Azi F, Ogbo FC, Nwankwegu AS, Odo MO, Anagboso MO (2016). Effect of Pre-treatment Methods on the Quality Characteristics of Stored Irvingia kernel. Br. Microbiol. Res. J. 14(5):1-7.
Crossref
|
|
|
|
|
Beuchat LR (2002). Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 4:413-423.
Crossref
|
|
|
|
|
Buck JW, Walcott RR, Beuchat LR (2003). Recent trends in microbiological safety of fruits and vegetables. Plant Health Progress 10:1094.
|
|
|
|
|
David OM, Aderibigbe EY (2010). Microbiology and Proximate Composition of Ogiri, Melon Seeds. New York. Sci. J. 3(4):18-27.
|
|
|
|
|
De Roever C (1998). Microbiological safety evaluations and recommendations on fresh produce. Food Contr. 9:321-347.
Crossref
|
|
|
|
|
European Commission-Scientific Committee on Food (EC-SCF) (2002). Risk Profile on the Microbiological Contamination of Fruits and vegetables Eaten Raw. Report of the Scientific Committee on Food, European Commission health and Consumer protection Directorate-general.
|
|
|
|
|
Enuijiugha VN (2009). Major Fermentative Organisms in some Nigerian Soup Condiments. Pak. J. Nutr. 8:279-283.
Crossref
|
|
|
|
|
Enuijiugha VN, Akanbi CT (2005). Compositional Changes in African Oil Bean (Pentaclethra macrophylla Benth) Seed Processing during Thermal Processing. Pak. J. Nutr. 4(1):27-31.
Crossref
|
|
|
|
|
Isu NR, Njoku HO (1997). An evaluation of the microflora associated with fermented African oil Bean (Pentaclethra macrophylla) seeds during ugba production. Plant Foods Hum. Nutr. 51: 145-157.
Crossref
|
|
|
|
|
Isu NR, Ofuya CO (2000). Improvement of the traditionally processing and fermentation of African oil bean (Pentaclethra macrophylla Bentham) into a food snack-ugba. Int. J. Food Microbiol. 59:235-239.
Crossref
|
|
|
|
|
Jideani IOA, Okeke CR (1991). Comparative Study of Microorganisms and Sensory Attributes of Condiments from the Fermentation of Different Seeds. Plant Food Hum. Nutr. 41:27-34.
Crossref
|
|
|
|
|
Njoku HO, Ogbulie JN, Nnubia C (1990). Microbiological Study of Traditional Processing of African Oil Bean (Pentaclethra macrophylla Benth) for Ugba Production. Food Microbiol. 7(1): 13-26.
Crossref
|
|
|
|
|
Mbata T, Orji MU (2008). Process optimization in the production and preservation of Ugba, a Nigerian fermented food. Int. J. Microbiol. 4:2-6.
|
|
|
|
|
Nwagu TN, Amadi C, Alakwe J (2010). Role of Bacteria Isolates in the Spoilage of Fermented African Oil Bean Seed Ugba. Pak. J. Biol. Sci. 13:497-503.
Crossref
|
|
|
|
|
Obeta JAN (1983). A note on the microorganisms associated with fermentation of seeds of the African oil bean tree (Pentaclethra macrophylla). J. Appl. Bacteriol. 54: 433-433.
Crossref
|
|
|
|
|
Odibo FJC, Nwabunia E, Osuigwe DI (1990). Biochemical changes during fermentation of Telfairia Seeds for Ogiri production. World J. Microbiol. Boitechnol. 6:425-427.
Crossref
|
|
|
|
|
Odibo FJC, Umeh AI (1989). Microbiology of Melon Seed Fermentation for Telfairia seeds for Ogiri Production. MIRCEN J. Appl. Microbiol. Biotech. 5:217-222.
Crossref
|
|
|
|
|
Odunfa SA (1981). Microorganisms associated with fermentation of African locust beans (Parkia filicoidea) during iru fermentation. J. Plant Foods 3:245-250.
Crossref
|
|
|
|
|
Odunfa SA (1985). Microbiological and toxicological aspects of fermentation of castor oil seeds for ogiri production. J. Food Sci. 50:1758-1764.
Crossref
|
|
|
|
|
Oranusi SU, Oguoma OI, Agusi E (2013). Microbiological quality assessment of foods sold in students cafeterias. Global Res. J. Microbiol. 3(1):1-7.
|
|
|
|
|
Oranusi US, Braide W (2012). Microbiological safety assessment of apple fruits (Malus domestica Borkh) sold in Owerri Imo State Nigeria. Adv. J. Food Sci. Technol. 4(2):97-102.
|
|
|
|
|
Sanni AI (1993). The need for process optimization of African fermented foods and beverages. Int. J. Food Microbiol. 18:85-95.
Crossref
|
|
|
|
|
Sanni AI, Ayernor GS, Sakyi-Dawson E, Sefa-Dedeh S (2000). Aerobic spore-forming bacteria and chemical composition of some Nigerian fermented soup condiments. Plant Foods Human Nutr. 55:111-118.
Crossref
|
|
|
|
|
Shuaib FMB, Ehiri J, Abdullahi A, Williams JH, Jolly, PE (2010). Reproductive health effects of aflatoxins: A review of the literature. Reproductive Toxicolol. 29:262-270.
Crossref
|
|
|
|
|
Strosnider H, Azziz-Baumgartner E, Banziger M, Bhat RV, Breiman R,
Crossref
|
|
|
|
|
Turner PC, Moore SE, Hall AJ, Prentice AM, Wild CP (2003). Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environ Health Perspect. 111:217-220.
Crossref
|
|
|
|
|
Wang J, Fung DY (1996). Alkaline fermented foods: A review with emphasis on pidan fermentation. Critical Rev. Microbiol. 22(2):101-138.
Crossref
|
|
|
|
|
World Health Organization (WHO) (2008). WHO Global Strategy for Food Safety: Safer Food for Better Health. Food Safety Issues. Geneva: WHO, 26p.
|
|
|
|
|
Williams J, Philips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal J (2003). Human Aflatoxicosis in Developing Countries. A review of toxicology, exposure, potential health consequences and interventions. Am. J. Clin. Nutr. 80:1106-1122.
|
|
|
|
|
Wu F, Khlangwiset P (2010). Health Economic Impact and cost-Effectiveness of Aflatoxin Reduction Strategies in Africa: Case Studies in Biocontrol and Postahrvest Interventions. Food Addit. Contam. 27:496-509.
Crossref
|
|
|
|
|
Zamora AF, Fields MI (1979). Nutrive uality of fermented cowpea (Virgna sinensis) and chickpeas (Cicer arietinum). J. Food Sci. 44:234.
Crossref
|
|
|
|
|
Zuber P, Nakano MM, Marahiel MA (1993). Peptide antibiotics. In Bacillns snbtilis and Other Gram-positive Bacteria, pp. 897-916. Edited by A. L. Sonenshein, J. A. Hoch & R. Losick. Washington, DC : American Society for Microbiology.
|
|