Full Length Research Paper
ABSTRACT
Chlorophenols are compounds used as intermediaries in manufacturing agricultural pesticides. Despite the efficiency on their targets, they are considered as environmental pollutant substances. This study assessed two chlorophenol substances, 2-chlorophenol and 2,4-dichlorophenol (2-CP, 2,4-DCP) for their cytogenetic, genotoxic, and nucleic acid content affects using Allium cepa and Vicia faba assay. In A cepa, 2-CP low dosages (0.625%) increased the mitotic index (MI), while high dosages (1.25 and 2.5%) decreased it; the high dosage (2.5%) increased the chromosomal aberration frequency (CAF). Treatment with 2,4-DCP showed that 2.6% was the most affected treatment, it decreased MI and increased CAF. In V. faba, both 2-CP and 2,4-DCP decreased MI and increased CAF. Types of chromosome anomalies scored after treatment with different concentrations were chromosome stickiness, disturbance, lagging chromosomes, anaphase and telophase bridge, and micronuclei. The contents of DNA and RNA in A. cepa were increased by 2-CP and 2,4-DCP, and decreased by the half dosage of 2-CP (1.25%); the RNA content varied directly proportional to DNA, while in V. faba, all tested concentrations of chlorophenols increased nucleic acids contents, and 1.3% was significantly affected. The results showed that the two substances have cytogenetic, genotoxic impact, and also have influence on the nucleic acid contents in both plants.
Key words: Cytogenetic, genotoxic, chlorophenols, Allium cepa, Vicia faba.
INTRODUCTION
Chlorophenols act as intermediaries in manufacturing agricultural chemicals, biocides, and dyes (Igbinosa et al., 2013). Although agricultural chemicals significantly affect disease control, they can also cause considerable environmental and health difficulties from production through disposal. Chlorophenols are considered environmental pollutants due to chemical and pharmaceutical industrial activities (Jensen, 1996; Czaplicka, 2004; Michalowicz and Duda, 2007; Igbinosa et al., 2013; Adeola, 2018). They enter the environment through various sources, such as industrial waste, pesticides, or by degrading complex chlorinated hydrocarbons (Igbinosa et al., 2013). In Finland, people’s exposure to contaminated drinking water with chlorophenols caused digestive tract infection, asthma, depression, and morbidity (Lampi et al., 2000). Chen et al. (2004) proposed that chlorophenols have cytotoxic and cell death mechanisms. Atuanya and Onuoha (2018) suggested continues monitoring of agricultural farmlands for the continuous exposure to pesticides that led to food contamination. Treatment with different chlorophenols caused various problems in cells, such as disturbance in the cell cycle, chromosome aberrations, and genetic material damage in other tested living systems. Researchers have documented these cases. Kü?ük and Liman (2018) found that treatment with different concentrations of 2-chlorophenol (2-CP) slightly decreased mitotic index (MI) and several types of chromosomal aberrations, such as disturbance, chromosome laggards, stickiness, and bridges on Allium cepa root tip cells.
Kü?ük and Liman (2018) discussed the effects of chlorophenols on genetic material and found that they could oxidize DNA bases in human lymphocytes and that pyrimidine bases were more strongly oxidized compared to purine ones. Also, the effects are not only confined to the parent material, but also to the transformation products. Benfeito et al. (2014) found that chlorophenoxy herbicide transformation products had inhibitory activity on DNA compared with the parent herbicides. Kü?ük and Liman (2018) concluded that 2-CP affected DNA at significant levels. Several studies have shown that chlorophenols affect DNA (Dimitroy and Gadeva, 2006; Tingting et al., 2017).
The most significant commercial chlorophenols are 2,4-DCP (Czaplcka, 2004), pentachlorophenol (PCP) (Michalowicz and Duba, 2007), 2,4,5-trichlorophenol (2,4,5-TCP) (Jensen, 1996), 2-CP (Balcke et al., 2008), and 4-CP (Abhilash and Singh, 2008).
2-CP is a chlorinated organic class used as an intermediate for synthesizing higher chlorinated congeners, certain dyes, herbicides, fungicides, and plastics (Kü?ük and Liman, 2018). Previous studies have confirmed the harmful effect of 2-CP. Dimitris et al. (2016) tested the impact of 2-CP on bacteria, fish, and human cells. They found that treatments can induce dose-dependent toxic and genotoxic effects.
2,4-DCP is a colorless crystalline solid, a chlorinated phenol derivative used as an intermediate for herbicide preparation of 2,4-dichlorophenoxyacetic acid. Previous studies of 2,4-DCP and its derivatives have revealed that while killing harmful organisms, it will upset the ecosystem and produce undesirable changes in higher organisms (Ajay and Sarbhoy, 1988); its destructive and toxic substance can be referred for their easy skin and epithelium penetration, thereby causing damage and necrosis (Michalowicz and Duda, 2007).
Higher plant assays are used in monitoring and detecting cytogenetic and mutagenic effects and have been recognized as excellent genetic models. The A. cepa assay is an efficient test for chemical screening for genotoxicity of environmental contaminants and has been widely used in studying genotoxicity of many pesticides, revealing that these compounds can induce chromosomal aberrations in root meristems of A. cepa (Ma et al., 1994; Fernandes et al., 2007).
Vicia faba bioassay has been used to study DNA damage and chromosomal and nuclear aberrations. The main benefits of using V. faba are accessibility during the year, easy to grow and handle, cell division frequency is fast, easy chromosomes scoring, less expensive, and more sensitive (Iqbal, 2016).
This study assesses 2-CP and 2,4-DPC effects on MI and CAF, and their impact on nucleic acid content using A. cepa and V. faba assay.
MATERIALS AND METHODS
(1) A. cepa bulbs were obtained from the local markets. Bulbs of A. cepa outer loose crusts and old roots were disposed, the running water with the help of a brush was used to remove the remaining sands particles to expose the root tips for different treatments.
(2) V. faba seeds were obtained from the local market. They were sterilized using sodium hypochlorite for 5 min, then presoaked for 24 h in distilled water.
Tested materials
(1) 2-CP of three different concentrations was prepared (0.625, 1.25, and 2.5%), and distilled water was used to prepare the solutions.
(2) 2,4-DCP of three different concentrations was also prepared (0.65, 1.3, and 2.6%), and distilled water was used to prepare the solutions.
Sample treatments
(1) A. cepa bulbs’ root zone of the onion bulb was submerged in a cup filled with distilled water until the roots reached 2 to 3 cm long; then, they were transferred to different concentrations of each substance, freshly prepared for different periods (8, 16, and 24 h). (2) The soaked seeds of V. faba were germinated in distilled water until the secondary roots reached 1 to 2 cm and were transferred to different tested concentrations of each substance, freshly prepared for different periods (8, 16, and 24 h).
The treated root tips of A. cepa and V. faba were cut and fixed in freshly prepared 3:1 (v/v) alcohol: glacial acetic acid for 24 h. For cytological preparations, the treated root tips were hydrolyzed in 1 N HCL 60°C for 7 min and were washed with distilled water and stained with 1% acetocarmine; five temporary slides were prepared using the squash technique, and two root tips on each slide were examined for the tested material effects on MI. The same slides were analyzed for the types and frequencies of chromosomal abnormalities.
Nucleic acids (DNA, RNA) isolation and measurement
Genetic material isolation was applied following the instructions of Hi Pura TM (Plant DNA isolation (CTAB Method) kit). The concentrations of isolated nucleic acids were measured by IMPLEN Nano Photometer® instruments, version NPOS 3.1c build13220.
Slides scoring and data analysis
Studying the slides: Slides were viewed under a light microscope (Phenix P H 50 DB047VU) using 40X objective lens immersion.
Mitotic index (MI): On one slide for each treatment, a total of 2000 cells were scored. The MI was expressed as the number of dividing cells per total cells scored.
Cytotoxicity: The MI of the treated cells for each dose of each material was compared with that of the negative control.
Genotoxicity test: Chromosomal aberrations per dose of each material were examined; the most representative ones for each structural abnormality were photographed using (Phenix micro-Image Analyzer Software 2008 En V2).
Statistical analysis
The percentages of MI and CAF of each tested dose were compared with those of the negative control using the SPSS 16.0 for Windows statistical package. A two-way analysis of variance (ANOVA) was used to determine the significance of the difference at P ≤ 0.05.
RESULTS
MI and CAF
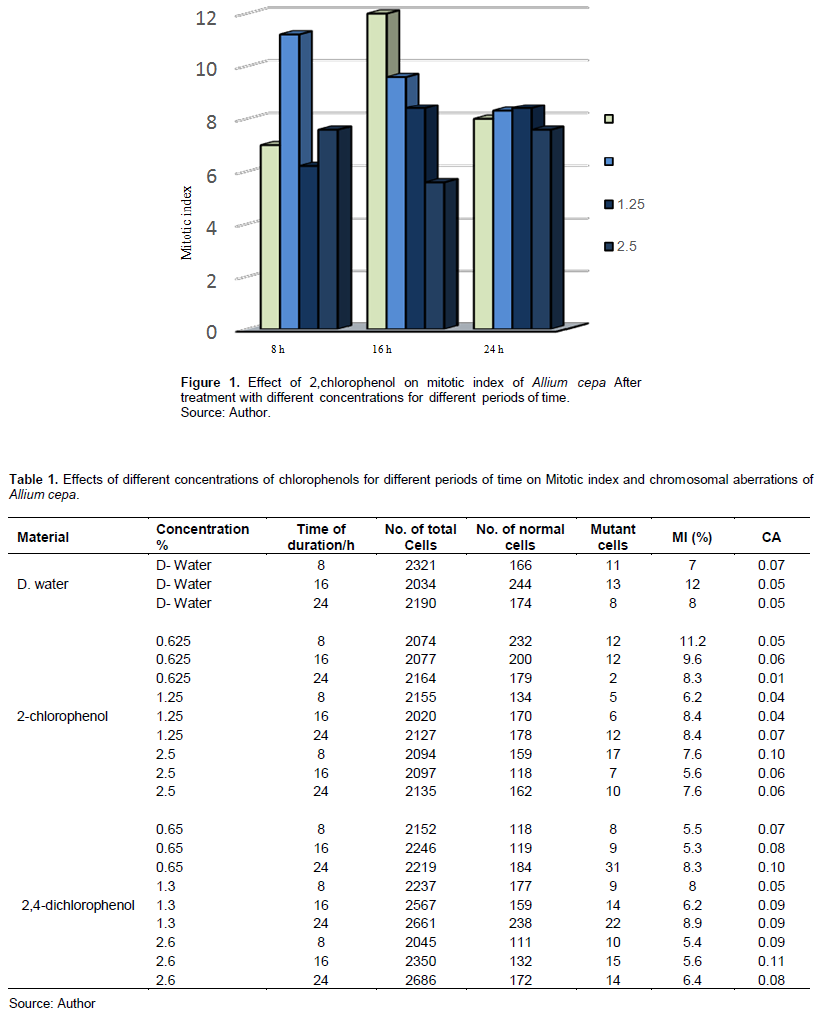
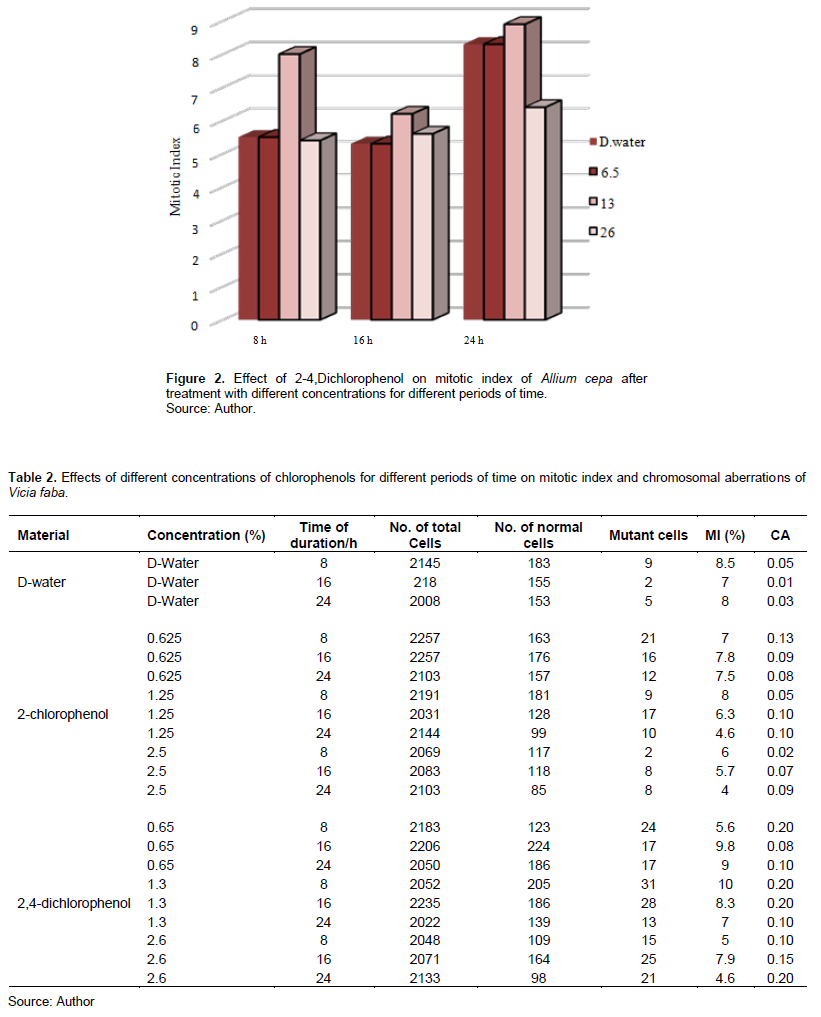
Tables 1 and 2 show 2-CP and 2,4-DCP effects on the MI in meristem cells of A. cepa and V. faba root tips.
Table 1 and Figure 1 show that 2-CP tested concentrations affected the MI of A. cepa root tip cells compared with the control. Treatment with 0.625 and 2.5% for 8 h increased the MI, and it was insignificant at p > 0.05, while other treatments decreased it; treatment with 2.5% for 16 h was significant at p < 0.05.
2,4-DCP also affected the MI in Figure 2 and treatment with 0.65 and 2.6% decreased the MI after 8-h exposure, and it was significant at p < 0.05, while treatment with 1.3% increased it and was insignificant at p > 0.05; also, treatment with 0.65 and 2.6% for 16 h decreased the MI of A. cepa root tip cells, and it was significant at p < 0.05.
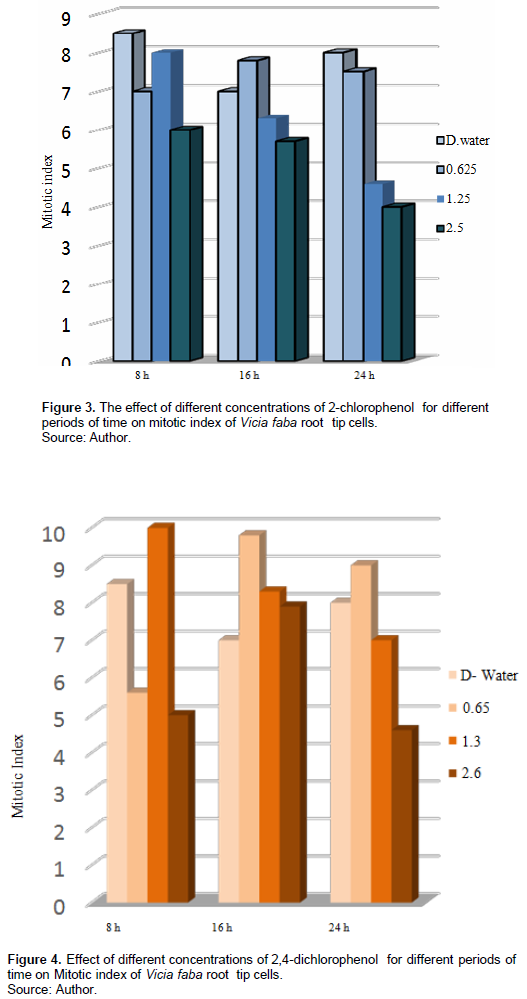
Table 2 and Figure 3 show the 2-CP effect on the MI on root tip cells of V. faba, different tested concentrations for different periods decreased the MI compared with the control; treatment with 0.625% increased the MI after 16 h exposure, and it was insignificant at p > 0.5, while treatment with 1.25% for 16 h and 2.5% for 24 h decreased the MI significantly at p < 0.05.
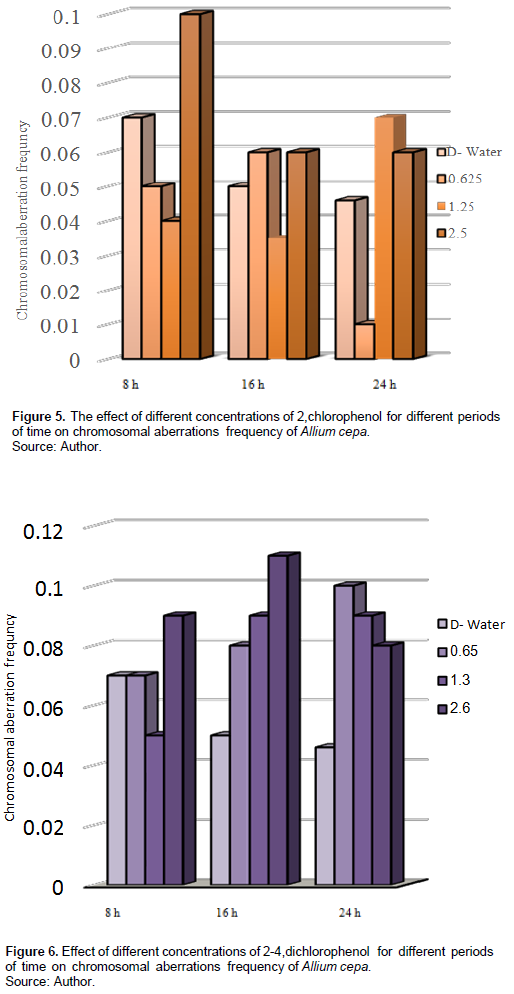
Figure 4 shows that the treatment with 0.65% of 2,4-DCP for 16 and 24 h increased the MI with 9.8 and 9, respectively, compared with the control (7, 8), and it was insignificant at p > 0.5; also, treatment with 1.3% for 8 and 16 h increased the MI with 10 and 8.3, respectively, compared with the control (8.5, 7), and it was insignificant at p > 0.5; treatment with higher concentration of 2.6% of 2,4-DCP for 16 h increased the MI insignificantly at p > 0.5. It decreased the MI with 4.6 compared with the control eight after 24 h, and it was significant at p < 0.5.
The tested materials affected the CAF compared with the control. Table 1 and Figure 5 show that treatment with different concentrations of 2-CP increased the CAF with 2.5% after 8 h, and 1.25 and 2.5% after 24 h, while with 0.625 and 2.5% after 16-h exposure, and they were insignificant at p > 0.05.
Figure 6 shows the effect of treatment with 2,4-DCP for different periods. It increased the CAF compared with the control in A. cepa root tip cells, and treatment with 0.65% for 24 h and
treatment with 2.5% for 16 h were significant at p< 0.05.
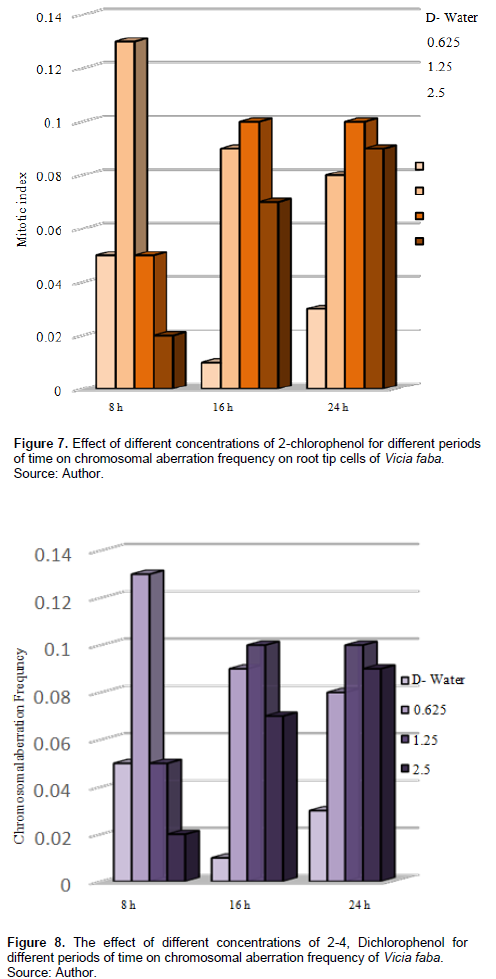
In V. faba, Table 2 and Figure 7 show that CAF increased after treatment with different concentrations for different periods compared with the control, treatment with 0.625% increased the CAF, also 0.13% after 8-h exposure and was significant at p < 0.05; treatment with 1.25% for 16 and 24 h increased the CAF with 0.1 each, and it was significant at P < 0.05.
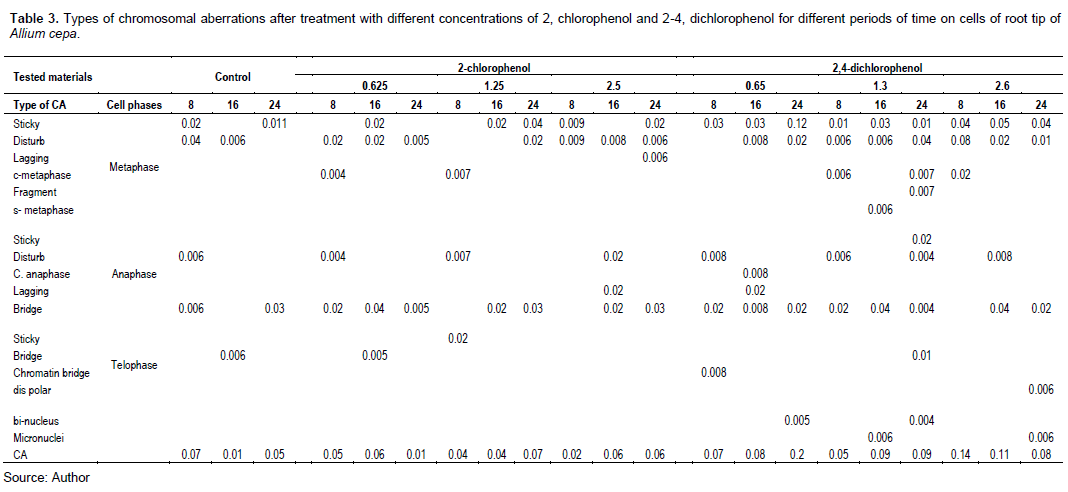
Figure 8 shows that treatment with different concentrations of 2,4-DCP affected the CAF. All the tested concentrations increased the CAF, and it was significant at p < 0.05 compared with the control.
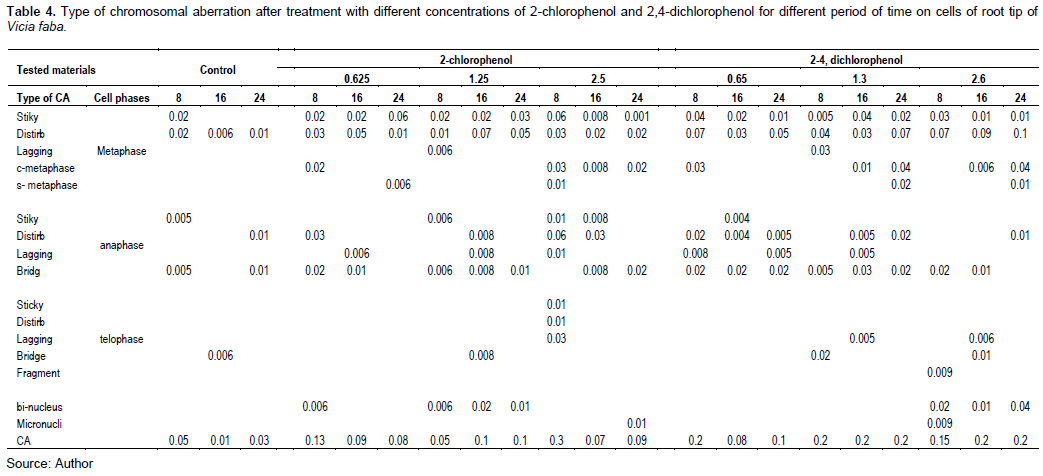
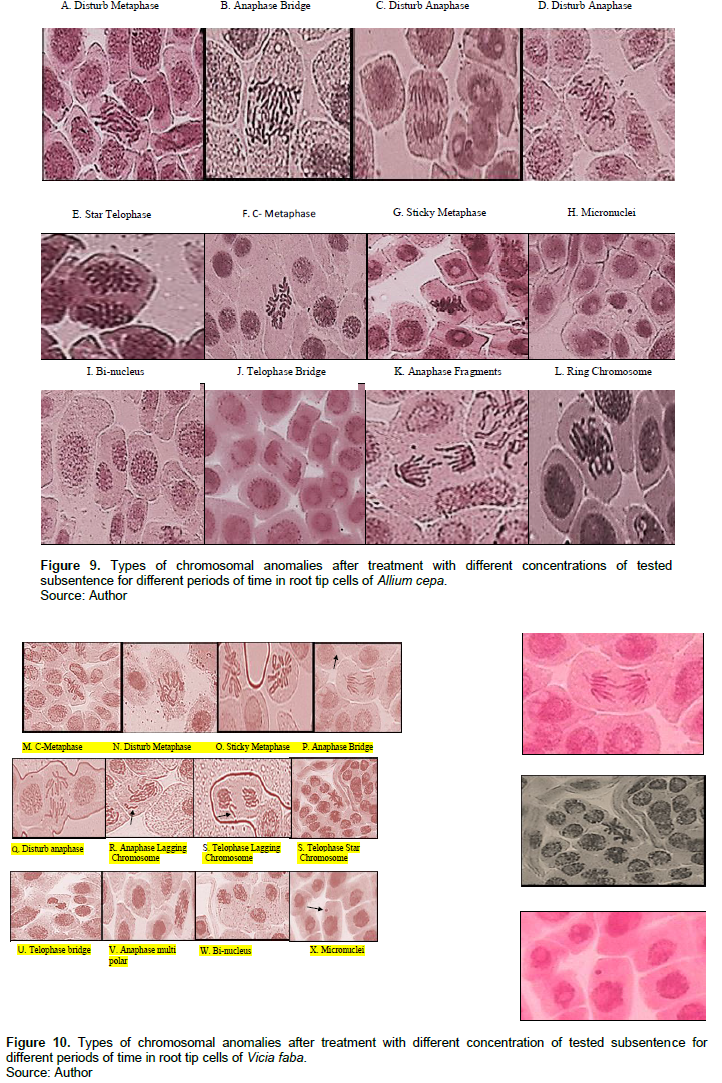
Tables 3 and 4 and Figures 9 and 10 show the types of scored chromosome anomalies, which were stickiness, C-metaphase, disturbance, anaphase bridge, micronuclei, lagging chromosome, disturbed polar, and bi-nucleic.
Nucleic acid content
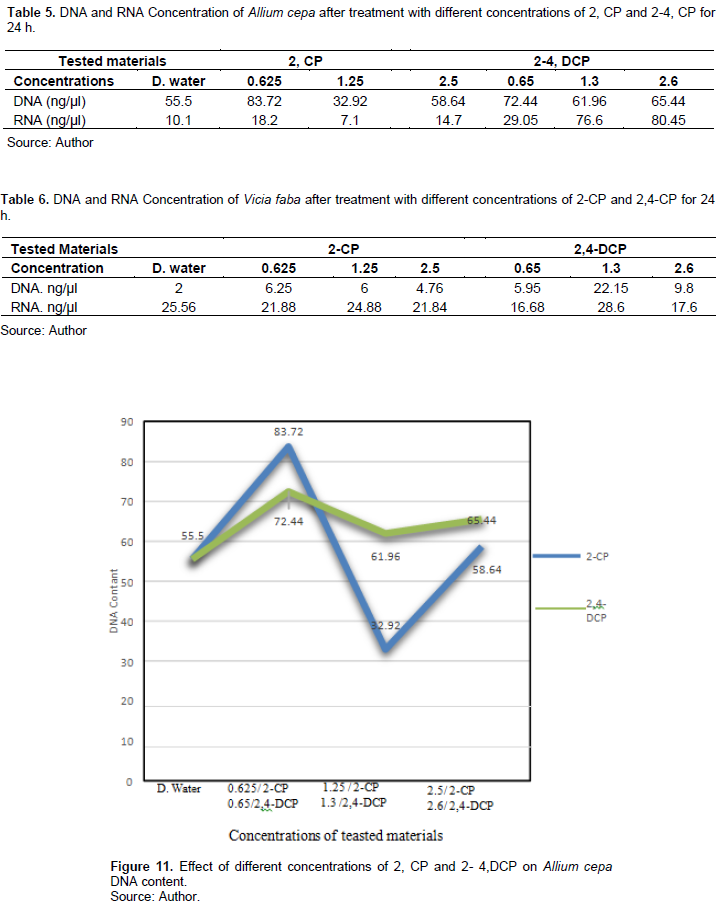
Table 5 and Figures 11 and 12 show 2-CP and 2,4-DCP effects on A. cepa DNA and RNA contents after treatment for 24 h. Treatments with 0.625 and 2.5% of 2-CP increased the DNA content (83.72, 58.64 ng/µl), respectively, compared with the control (55.5 ng/µl), and it was significant at p < 0.05, while treatment with 1.25% decreased the DNA content (32.92 ng/µl), and it was significant at p < 0.05. Also, treatment with 0.625 and 2.5% increased the RNA content (18.2, 14.7 ng/µl), respectively, compared with the control 10.1 ng/µl, while treatment with 1.5% decreased the RNA content to 7.1 ng/µl, and these effects were significant at p < 0.05.
2,4-DCP concentrations increased the DNA content after treatment for 24 h (72.44, 61.96, and 56.44 ng/µl), respectively, compared with the control 55.5 ng/µl, and the effect was significant at p < 0.05. Different treatments significantly affected the RNA content; it increased (29.05, 76.6, and 80.45 ng/µl) compared with the control 10.1 ng/µl.
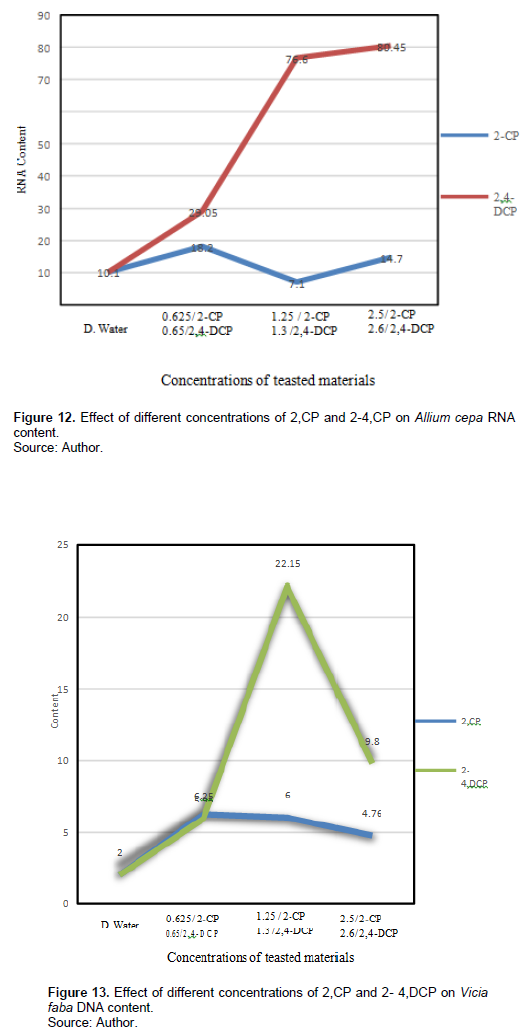
Table 6 and Figures 13 and 14 show the effect of different treatments of 2-CP and 2,4-DCP on DNA and RNA contents of V. faba root tip cells. The 2-CP tested concentrations increased the DNA content (6.625, 6, and 4.76 ng/µl), respectively, and they were significant at p < 0.05; also, treatment with 2,4-DCP increased the DNA content (5.95, 22.15, 9.8 ng/µl) compared with the control 2 ng/µl, and it was significant at p < 0.05.
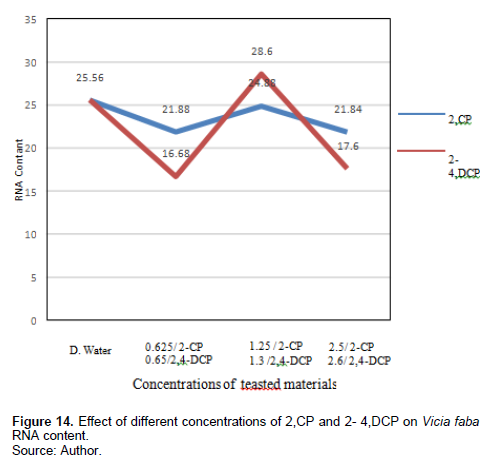
RNA contents were decreased by the tested concentrations of 2-CP after 24-h exposure (21.88, 24.88, and 21,84 ng/µl), respectively, compared with the control 25 ng/µl. Treatment with 0.65% and 2,4-DCP reduced RNA content (16.68 and 17.6 ng/µl), respectively, while treatment with 1.3% increased the RNA content (28.6 ng/µl) compared with the control 10.1 ng/µl, and this effect was significant at p < 0.05.
DISCUSSION
Mitotic Index (MI) and Chromosomal Aberration Frequency (CAF)
The mitotic index, MI (percentage of cells in mitosis at any time) offers a measure of the capacity of cells to divide and of the rate of cell division (Campbell, 1983). The result showed that treatment with different concentrations of 2-CP and 2,4-DCP for different periods decreased the MI and increased the CAF compared with the control. This decrease in MI may be due to the MI depressive action of the tested materials interfering in the regular cell cycle (Chandra et al., 2002; ?NCEER et al., 2003; Sharma and Vig, 2012; Liman et al., 2015; Hoseiny-Rad and Aivazi, 2020). Hidalgo et al. (1985) suggested that DNA polymerase and other enzymes are among the tested material targets that induced antimitotic effects. Lehnen et al. (1990) found that MI decrease is attributed to the inhibition of DNA synthesis and formation of irregular and disorganized phragmoplasts. Öcal and Ero?lu (2012) suggested that the MI decrease may be due to the possible cellular death or delay in cell proliferation. Also, Hansch et al. (2000) concluded that phenol toxicity is related to free radical formation. A similar result was found by Dimitris et al. 2016, Kumar et al. (2010), Lone et al. (2013), and Kumar and Srivastava (2015).
Chromosomal aberration assay for cytological aberrations is a useful and sensitive test for detecting genotoxins (Sheila, 2000).
The results reveal that treatment with 2-CP and 2,4-DCP caused several types of chromosomal aberrations, and the most noticed chromosome abnormalities that appeared after treatment with 2-CP were stickiness, disturbed, and anaphase bridge; other types of abnormalities but in low percentage were lagging chromosomes, fragment, C- metaphase, S-anaphase, bi-nucleus, micronuclei, and irregular poles. 2,4-DCP strongly affected spindle function, causing the appearance of disturbance, C-metaphase, S-anaphase, and laggards. This effect was caused by binding to a protein subjected to spindle microtubule (Brinkley et al., 1985; Pavlica et al., 1991). Chromosome stickiness can be due to the effect of the chromosomal proteins or disturbances in the functioning of specific non-histone proteins essential for chromatid separation and segregation (Gaulden, 1987); also, chromosome stickiness may occur because of chromosomal protein, which is attributed to the irregular folding of chromosome fibers or the action on the polymerization process (El-Ghamery et al., 2000). Mahakhode and Somkuwar (2013) suggested that it may occur due to the sudden contraction of some spindle fibers. Kumar and Srivasatava (2015) reported that scattering (disturbance) and spindle dysfunction were attributed to the loss of the spindle fibers, microtubules.
C-mitosis during metaphase and anaphase were also scored, and this type of abnormality can be due to the effect of the tested material on spindle fibers. Morejohn and Fosket (1986) proposed that C-mitosis appears because of mitosis blocking at metaphase and depolymerizes spindle microtubules; a similar result was found by Dimitrov and Gadeva (2006).
Chromosome fragments appeared because of chromosome breaks (chromatids). Kumar and Singh (2004) reported that chromatids’ breakage had been linked to DNA synthesis, which is sensitive to many chemicals. Kumar et al. (2010) concluded a comparable result.
The chromosome bridge was formed from the sticky behavior of chromosomes that cannot move toward pole regions at anaphase (Kumar and Rai, 2007), it may also be due to the chromosomal instability (Ganem et al., 2009); a similar result was found by Kumar and Srivastava (2015). Sinha (1989) suggested that chromosomebridge was attributed to the formation of dicentric chromosomes due to breakage and reunion.
For micronuclei definition and scoring, the micronuclei criteria by Tolbert et al. (1991) were followed. The failure of the movement of lagging chromosomes increases micronuclei (Kumar and Dubey, 1998). Dichlorophenol is active in the G1 and S phases of the cell cycle (Arzt et al., 1989; Lone et al., 2013).
Nucleic acid content
The nucleic acid biosensor can be used to detect chemical species (Palchetti and Mascni, 2008). Treatment with different concentrations of 2-CP for 24 h affected the DNA content compared with the control. The lowest and highest concentrations of 2-CP increased the DNA content of A. cepa, and the half concentration decreased it, while treatment with 2,4-DCP concentrations increased the content of DNA. RNA was also affected by DNA concentration; RNA content was directly proportional to DNA content.
In V. faba treatments with different concentrations of 2- CP and 2,4-DCP, DNA content was increased; treatments with different concentrations of 2-CP decreased the RNA content compared with the control. While treatment with half concentration of 2,4-DCP increased it.
The increased DNA content may be due to the low concentration dosage. El-Hadary and Gyuhwa (2013) suggested that herbicides in low doses act as growth regulators, while a decrease in DNA may be due to the effect of dosage on cells biochemical activities. Sharma et al. (2017) concluded that pesticides cause oxidative stress in plants by producing reactive oxygen species (ROS). Plants resist pesticide toxicity by activating the internal antioxidation defense system, which includes an antioxidative enzyme and non-enzymatic antioxidation. A similar result was found by Kuckl and iman (2018) when A. cepa was treated with 2-CP; it may also be due to the action of the tested concentrations, which was insufficient to counteract the oxidative damage induced by the overproduction of ROS (Fernades et al., 2020). Duchnowicz et al. (2002) explained that the toxicity of higher concentrations of 2,4-DCP decreases ATPase activity. 2,4-DCP induced ROS overproduction and DSBs, showing that ROS accumulation and GSH depletion are involved in 2,4-DCP, causing DNA damage in fish (Huang et al., 2018). A similar result was found by Tingting et al. (2017) in membrane, which induced a change in ion transport and disruption of both sides of the membrane, agreeing with the result of Bukowska (2006; Michalowice and Majsterek 2010).
CONCLUSION
The tested materials (2-CP and 2,4-DCP) showed that different concentrations affected the MI and CAF in both plants (A. cepa and V. faba). Several types of chromosome anomalies were scored, such as stickiness, disturbance, anaphase bridges, micronuclei, and binuclei. Although nucleic acid contents (DNA and RNA) increased after treatment with different concentrations (0.625 and 2.5%) of 2-CP and (0.65, 1.3, and 2.6%) of 2,4-DCP, they decreased by 1.25% of 2-CP. The increase in DNA and RNA content may be due to protective action by protein production that allows the plant to continue the biological process normally under chemical stress, or it may be due to the interference of chemicals with the cell cycle by disturbing the normal flow of the process, leading to DNA content accumulation and decreasing cell division MI. The tested materials have cytogenetic, genotoxic, and also influence in nucleic acid contents in A. cepa and V. faba plants.
ACKNOWLEDGEMENT
The author appreciates Dr. Hesham El. Komy and Dr. Mohammed Al. Shamlah in Science Research Units of College of Science for their valuable support for this study.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Abhilash PC, N Singh (2008). Distribution of hexachlorocyclohexane isomers in soil samples from a small-scale industrial area of Lucknow, north India, associated with Indian production. Chemosphere 73(6):1011-1015. |
|
|
Adeola AO (2018). Fate and toxicity of chlorinated phenols of environmental implications: A review. Medicinal and Analytical Chemistry International Journal 2(4):1-8. |
|
|
Ajay KJ, Sarbhoy RK (1988). Cytogenetic studies on the effects of some chlorinated pesticides III. Concluding remarks. Cytologia 53(3):427-436. |
|
|
Arzt ES, Castelo SF, Diaz A, Finkielman S, Nahmod VE (1989). The muscarinic pilocarpine inhibits DNA and interferon- γ cells. synthesis in peripheral blood mononuclear. International Journal of Immunopharmacology 11(3):275-281. |
|
|
Atuany A, El; Onuoha T (2018). Level of Organochlorine Pesticide Residues in selected Consumable Vegetables commonly sold im Benin City Markets. Journal of Applied Sciences and Environmental Management 22(10)1625-1630. |
|
|
Balcke GU, Wagener S, Kiesel BM, Benndorf M, SchlÖmann, Vogt C (2008). Kinetics of chlorobenzene biodegradation under reduced oxygen level. Biodegradation 19(4):507-518. |
|
|
Benfeito S, Tiago S, Garrido J, Andrade PB, Sottomayor MJ, Broges F, Garrido EM (2014). Effects of Chlorophenoxy Herbicide and Their Main Transformation Products on DNA Damage and Acetylcholinesterase Activity. BioMed Research Intranational. |
|
|
Brinkley BR, Tousson A, Valdivia M (1985). The kinetochore of mammalian chromosomes: structure and function in normal mitosis and aneuploidy, in: V.L. Dellarco, P.E. Votek and A. Hollaender (Eds), Aneuploidy, Etiology and Mechanisms, Plenum, New York, 243-265. |
|
|
Bukowska B (2006). Toxicity of 2,4-Dichlorophenenoxyacetic acid- Molecular Mechanisms. Polish Journal of Environmental Studies 15(3):365-374. |
|
|
Campbell RD (1983). Mitotic Index. In: Lenhoff H.M. (eds) Hydra: Research Methods. Springer, Boston, MA. |
|
|
Chandra BR, Saharan RP, Sareen P K (2002). Clastogenic effect of trifluralin in Vici faba. Journal of Cytology and Genetics 3:201-203. |
|
|
Chen J, Jiang J, Zhang F, Yu H, Zhang J(2004). Cytotoxic environmentally relevant chlorophenols on 1929 cells and their mechanisms. Cell Biology and Toxicology 20(3):183-196. |
|
|
Czaplicka M (2004). Sources and transformation of chlorophenols in the natural environment. Science of the Total Environment 322(1-3):21-39. |
|
|
Dimitris V, Antonopoulou M, Konstantioou KI (2016). Evaluation of toxicity and genotoxicity of 2-chlorophenol on bacteria, fish and human cells. The Science of total Environment 551-552 (1):649-566. |
|
|
Dimitrov BD, Gadeva PG (2006). Comparative genotoxicity of the herbicides Roundup, Stomp, and Reglone in plant and mammalian test system. Mutagenesis 21(6):375-382. |
|
|
Duchnowicz P, Koter M, Duda W (2002). Damage of erythrocyte by phenoxyacetic herbicides and their metabolites. Pesticide Biochemistry and Physiology 74(1):1-7 |
|
|
El-Ghamery AA, El-Nahas AI, Mansour MM (2000). The action of atrazine herbicides as an indicator of cell division on chromosomes and nucleic acids content in root meristem of Allium cepa and Vicia faba. Cytologia 65(3):277-287. |
|
|
El-Hadary MH, Chung G (2013). Herbicides - A Double Edged Sword. Licensee Intech. Open. This chapter is distributed under the terms of the Creative Commons Attribution 3.0 License. |
|
|
Fernandes TCC, Mazzeo DEC, Marin-Morales MA (2007). Mechanism of micronuclei formation in polyploidizated cells of Allium cepa exposed to trifluralin herbicide. Pesticide Biochemistry and Physiology 88(3):252-259. |
|
|
Lone IN, Shukla MS, Charles Richard JL, Peshev ZY, Dimitrov S, Angelov D (2013). Binding of NF-κ B to nucleosomes: effect of translational positioning, nucleosome remodeling and linker histone H1. PLoS Genetics 9(9):e1003830. |
|
|
Ganem NJ, Godinho SA, Pellman D(2009). A mechanism linking extra centrosomes to chromosomal instability. Nature 460(7252):278-282. |
|
|
Gaulden ME (1987). Hypothesis: Some mutagens directly alter specific chromosomal proteins (DNA topoisomerase II and peripheral proteins) to produce chromosomes stickiness, which causes chromosome aberrations. Mutagenesis 2(5):357-365. |
|
|
Hansch C, McCarns S, Smith C, Dodittle D (2000). Comparative QSAR evidence for a free-radical mechanism of phenol-induced toxicity. Chemico-Biological Interactions 127(1):61-72. |
|
|
Hidalgo A, Gonzalez-Reyes JA, Navas P, Garcia-Herduge G (1985). Abnormal mitosis and growth inhibition in Allium cepa root induced by Propham and chlorpropham. Cytobios 57(228):7-14. |
|
|
Hoseiny-Rad M, Aivazi A (2020). Biochemical and cytogenetic of Imazethapyr on Cicer arietinum L. Journal of Applied Biology and Biotechnology 8(2):73-77. |
|
|
Huang D, Zhang X, Zhang C, Li H, Li D, Hu Y, Yang F, Qi Y (2018). 2,4-Dichlorophenol induces DNA damage through ROS accumulation and GSH depletion in goldfish Carassius auratus. Environmental and Molecular Mutagenesis 59(9):798-804. |
|
|
Igbinosa EO, Odjadjare EE, Chigor VN, Igbinosa IH, Emoghene AO, Ekhaise FO, Igiehon NO, Idemudia OG (2013). Toxicological Profile of Chlorophenols and their derivative in the Environment: The public health respective. The Scientific World Journal 460215.P:11. |
|
|
Inceer H, Ayaz S, Beyazo?lu O, ?entürk E (2003). Cytogenetic effects of copper chloride on the root tip cells of Helianthus annus L. Turkish Journal of Biology 27(1):43-46. |
|
|
Iqbal M (2016). Vicia faba bioassay for environmental toxicity monitoring: A review. Chemosphere 144:785-802. |
|
|
Jensen J (1996). Chlorophenols in the terrestrial environment. Reviews of Environmental Contamination and Toxicology 146:25-51. |
|
|
Kumar S, Dubey DK (1998). Effects of gamma rays, EMS and DES on meiosis in Lathyrus sativus. Journal of Cytology and Genetics 33(2):139-147. |
|
|
Kumar G, Rai P (2007). Genotoxic potential of mercury and cadmium in soyabean. Turkish Journal of Biology 31(1):13-18. |
|
|
Kumar G, Singh V (2004). Studies in MI and M2 generations of azide induced medium-strong desynaptic mutant of pearl millets. The Nucleus 47:159-163. |
|
|
Kumar G, Srivastava A (2015). Genotoxicity of herbicide Ingredients Glyphosate and Atrazine on root Meristem of Buckwhea (Fagopyrum. esculentum Moench). Jordan Journal of Biological Science 8(3):2221-226. |
|
|
Kumar S, Arya Sk, Roy BK, Singh AK (2010). The effects of 2,4-dichlorophenoxy acetic acid and isoproturon herbicides on the mitotic activity of wheat (Triticum sativum L.) root tips. Turkish Journal of Biology 34(1):55-66. |
|
|
Kü?ük D, Liman R (2018). Cytogenetic and genotoxic effects of 2-chlorophenol on Allium cepa L. root meristem cells. Environmental Science and Pollution Research 25(36):36117-36123. |
|
|
Lampi P, Vohlonen I, Tuomisto J, Heinonen OP (2000). Increase of specific symptoms after long-term use of chlorophenol polluted drinking water in a community. European Journal of Epidemiology 16(3):245-251. |
|
|
Lehnen JR LP, Vaughan MA, Vaughn KC (1990). Tertbutyl affects spindle microtubule organizing center. Journal of Experimental Botany 41(5):537-546. |
|
|
Liman R, Ci?erci ?H, Öztürk NS (2015). Determination of genotoxic effects of Imazethapyr herbicide in Allium cepa root cells by mitotic activity, chromosome aberration, and comet assay. Pesticide Biochemistry and Physiology118:38-42. |
|
|
Lone MI, Nazam N, Shaikh S, Ahmad W (2013). Genotoxicity of an organochlorine pesticide dichlorophene by micronucleus and chromosomal aberration assay using bone marrow cells of Rattus norvegicus. Caryologia 66(4):296-303. |
|
|
Ma TH, Cabera GL, Cebulska Wasilewska A, Chen R, Loarca F, Vandererg AL, Salamone MF (1994). Tradescantia stamen hair mutation bioassay. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 310(2):211-220. |
|
|
Mahakhode RH, Somkuwar SR (2013). Mitotic abnormalities induced by glyphosate in Pasoralea corylifolia. International Journal of Current Pharmaceutical Research 5(1):46-48. |
|
|
Michalowics J, Duda W (2007). Phenols- Source and toxicity. Polish Journal of Environmental Studies 16(3):347-362. |
|
|
Michalowicz J, Majsterek I (2010). Chlorophenols, chlorocatechols and chloroguaiacols induce DNA base oxidation in human lymphocytes. Toxicology.268(3):171-175. |
|
|
Morejohn LC, Fosket DE (1986). Tubulinsfrom plants fungi and protests; a review. In Shay J.W. (ed), cell ND Molecular biology of the Cytoskeleton. Plenum Publishing Crop, New York, pp. 257-329. |
|
|
Öcal A, Ero?lu HE (2012). In vitro cytogenetic effects of Hypericum heterophyllum in human peripheral blood lymphocytes. Bangladesh Journal of Pharmacology 7(1):36-41. |
|
|
Palchetti L, Mascn M (2008). Nuclei Acids Biosensor for Environmental Pollution Monitoring. The Analyst 133(7):846-845. |
|
|
Pavlica M, Papes D, Nagy B (1991). 2,4Dichlorophenoxyacetic acid causes chromatin and chromosome abnormalities in plant cells and mutation in cultured mammalian cells. Mutation Research 263(2):77-81. |
|
|
Sharma A, Kumar V, Thukral AK, Bhardwaj R (2017). Responses of Plants to pesticides Toxicity: An Overview. Planta Daninha 37:1-12. |
|
|
Sharma S, Vig AP (2012). Genotoxicity of Atrazine, Avenoxan, Diuron and Quizalofop-P-ethyl herbicides using the Allium cepa root chromosomal aberration assay. Terrestrial and Aquatic Environmental Toxicology 6(2):90-95. |
|
|
Sheila M, Galloway (2000). Cytotoxicity and chromosome aberrations in vitro: Experience in industry and the case for an upper limit on toxicity in the aberration assay. Environmental and Molecular Mutagenesis 35(3):191-201. |
|
|
Sinha SP (1989). Genotoxicity of Pesticides. In Perspective in Cytol, Manna G. K. (Ed.) Genetics, New Delhi. |
|
|
Tingting G, Jiangyuan H, Yongmei G, Xueyan ML, Chen Z, Naeem S, Dejun H (2017). The toxic effects of chlorophenols and associated mechanism in fish. Aquatic Toxicology 184:78-93. |
|
|
Tolbert PE, Shy CM, Allen J (1991). Micronucleus and other nuclear anomalies in buccal smears: A field test in snuff users. American Journal of Epidemiology 134(8):840-850. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0