ABSTRACT
The aim of this study was to compare the embryonic developmental rates in the Southern African cattle breeds. To do this, cryopreserved semen straws from Nguni, Bonsmara, and Boran bulls were thawed at 38°C and evaluated for sperm motility characteristics using Sperm Class Analyser (SCA). The fertilizing ability of frozen/thawed sperm was evaluated by performing artificial insemination (AI) and in vitro fertilization (IVF). For AI, superovulated cows were inseminated with frozen/thawed semen and then further evaluated for embryo development. For IVF, oocytes from the respective cows were retrieved using ovum pickup, and then matured. Following maturation, oocytes were co-incubated with semen for 6 h. In the Nguni breeds, the IVF method of embryo production was mildly superior to the in vivo method at the morula stage while the Bonsmara breed revealed the opposite effect at both the morula and blastocyst stages. In the Boran breed, the IVF method was highly superior with the in vivo method at the 8-cell stage while the opposite effect was observed at the blastocyst stage of embryonic development. This study suggests that the Boran breed is less susceptible to loss of embryonic development as compared to the Nguni and Bonsmara breeds.
Key words: Nguni, Bonsmara, Boran, embryo, beef breeds, motility, artificial insemination, in vitro fertilization.
The success of the Southern African regional beef industries depends on its indigenous cattle beef breeds for their sustainability and competitiveness in the global stage (Morgan et al., 1991; Mckenna et al., 2002; Scholtz and Theunissen, 2010). Among the Southern African regional indigenous cattle beef breeds, the Nguni, Bonsmara (a composite breed) and Boran have great meat quality attributes, such as, good carcass qualities
with carcass marbling and meat tenderness which are favourable to consumers (Scholtz, 1988; Strydom et al., 2008; Scholtz and Theunissen, 2010). All these breeds possess survival traits that are suitable for local conditions including tolerance to diseases and harsh environmental conditions. In addition, they possess superior growth and reproductive performance and are outstanding beef breeds (Strydom et al., 2008).
Despite their superior qualities, instances of costly reproductive failures occur as a result of a number of factors that interfere with conception or cause the loss of foetuses in cycling females (
Mokantla et al., 2004). Artificial insemination (AI) has often been used in our local beef industries to increase production using fresh or frozen/thawed semen (Ferraz et al., 2012). The
in vivo fertilizing capacity of frozen/thawed semen has always been suspect because it is influenced not only by semen quality and fertilizing ability but also by the respective recipient cow fertility related issues (Dalton, 2011). Hence, the efficiency of
in vivo fertilization has become a pressing issue worth investigating in our local beef breeds.
Moreover, the investigation of the in vitro fertilization sufficiency using frozen/thawed semen has revealed variable outcomes in embryo formation and development, due to poor semen quality (Sudano et al., 2013). It has been previously demonstrated that in frozen/thawed semen about 50% of sperm cells are rendered immotile following thawing and the fertilizing ability of such semen is significantly lower leading to decreased fertilization rates (Watson, 2000). However, advances in semen cryopreservation technologies, such as, the use of percoll gradient and centrifugation, promises to improve post-thaw semen qualities and insemination rates as shown by improved fertilizing rates during AI and IVF (Oliveira et al., 2012). Others have argued that reactive oxygen species are higher in such semen resulting in poor 2-4 cell and 8-cells embryonic developmental stages and poor blastocyst inner cell mass formations (Oliveira et al., 2012; Arias et al., 2017).
As a result of these observations, this study was conducted to compare and investigate the embryonic developmental rates in our Southern African Nguni, Bonsmara and Boran beef breeds using in vivo fertilization and IVF as an attempt to address some of the reproductive failures in these breeds. In addition, this study was conducted to establish any relationships or interactions between the semen qualities, embryo production method (AI or IVF) and embryo production rates in these local beef cattle breeds.
Animals
All animals used in this study are aged between 24 and 36 months and are kept at a quarantine area with access to water at all times. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Animals under the guidelines of the Agricultural Research Council, Animal Production Institute Animal Ethics Committee (APIEC16/029). Bulls were fed on eragrostis at ad lib. A total of sixty (20 Nguni; 20 Bonsmara; 20 Boran) non-pregnant, healthy cycling cows were used as donors and evenly distributed for two embryo production methods namely, ovum pick-up and embryo flushing. The donor cows were feeding on 7 to 10 kg Lucerne, ad lib Eragrostis, 1.5 kg of Afgri® embryo concentrate per day.
Evaluation of sperm motility characteristics
A total of 15 bulls (5 Nguni; 5 Bonsmara; 5 Boran) cryopreserved bull semen straws were thawed in warm (38°C) sterile water. Percoll gradient subjected semen was generously supplied by Embryo Plus (Embryo Plus, Brits, South Africa). This percoll gradient and centrifugation were used to separate live and motile sperm cells from the immotile and dead sperm cells and thus enrich the live and highly motile sperm population (Oliveira et al., 2012). To analyse for semen quality, a drop of about 10 µl semen was placed on a microscopic slide and covered with a microscopic cover slip. Thereafter, motility characteristics such as total motility, progressive motility, non-progressive motility, rapid motility, medium motility, slow and static motility were evaluated using a sperm class analyser (SCA).
AI, superovulation and embryo flushing
A total of 30 donor cows (10 Nguni; 10 Bonsmara; 10 Boran) were superovulated according to the method described by Pontes et al. (2009) with slight modifications on dosage. Briefly, a controlled internal drug release (CIDR®) (1.9 g/ml, Pfizer (Pty) ltd., Sandton, Republic of South Africa, RSA) was placed into the vagina of each cow of the three different breeds accompanied by intramuscular (i.m) injection of estradiol benzoate (1 g/ml Pfizer (Pty) ltd., Republic of South Africa) on day 0. An i.m of Cloprostenol Sodium (263 μg/ml, Estrumate®, Isando, RSA) was administered (i.m) to the cows after CIDR® removal on day 8 of oestrous synchronization followed by i.m injection of half the original dosage of estradiol benzoate on day 9. Heat was observed with the aid of heat mount detectors on day 9 (Kamar®, RSA). Day 0 was repeated by inserting a new CIDR three days after heat observation. On day 4, two injections of Follicle Stimulating Hormone (FSH), Folltropin-V® (20 mg, Armidale, Australia) were administered at 12 h intervals initiated for four days on a decreasing dosage, plus two injections of estrumate 12 h apart on the last two days of Folltropin®. Then cows were inseminated twice (12 and 24 h) after detection of standing oestrous with frozen/thawed semen from Nguni, Bonsmara and Boran bulls. Thereafter, embryo recovery was performed seven days after AI, whereby an epidural anaesthesia (lignocaine) was performed with a standard non-surgical technique to flush the uterine horns using a three way folley catheter. Retrieved embryos from the breeds studied were transferred into an embryo filter containing holding medium and evaluated using a stereo-microscope (Olympus SZ40, Olympus, Japan). Embryos were evaluated for embryo development (2-4 cells, 8-cell, Morula, Blastocyst).
Ovum pickup
Ovum pick up was performed as described by Petyim et al. (2000) with a minor modification. A total of 30 normal cycling donor cows (10 Nguni; 10 Bonsmara; 10 Boran) were restrained in a crush pen then given an epidural injection (Lignocaine) on the head of the tail. Thereafter, the rectum was emptied and the vulva was cleaned thoroughly with 70% alcohol. Following cleaning, the transducer was advanced into the external of the cervix. Thereafter, ovaries were held through the rectum and positioned over the transducer face so that the targeted follicle is transacted by the built in puncture line on the ultrasound monitor, which represented the projected needle path. When the targeted follicles were stabilized on the puncture line, the needle was inserted in the guide and advanced through the vaginal wall and into the follicle antrum. Follicular fluid was then aspirated using continuous negative pressure (about 95 mmHg) then transferred into the laboratory for oocytes searching under the stereo microscope.
IVF and Embryo production
In vitro maturation, fertilization and oocyte culture were done using procedures as described (Huang et al., 2001; Nazem et al., 2016) with slight modification on the use of bovine follicular fluid and hormonal concentrations. Briefly, the cumulus oocytes complexes (COCs) were matured for 24 h in TCM-199 (Gibco, Grand Island, NY) consisting of 10% FBS, 10% follicular fluid, 10 µg/ml Leutenizing Hormone, 1 µg/ml prostaglandin E2 and 1 µg/ml FSH under humidified atmosphere of 5% CO2 at 38.5°C. Following maturation, oocytes were fertilized in 100 µl drops of frozen-thawed percoll gradient subjected semen for 18 h at 38.5°C. Thereafter, oocytes were cultured in synthetic oviductal fluid (SOF) supplemented with bovine serum albumin (BSA) and incubated at 38.5°C in 5% CO2 for seven days.
Embryo staining
Fixing and staining were done based on methods described (Hossaini et al., 2007; Nazem et al., 2016). Briefly, seven days following culture, a sample of embryos were fixed in 4% paraformaldehyde for 48 h then later stained in 50 µg/mL of Hoechst 33342. Thereafter, embryos were placed individually on a microscope glass slide and covered with a coverslip and then evaluation under fluorescence microscope (Olympus-BX51TF).
Statistical analysis
Cleavage and blastocyst formation data were analysed by ANOVA. Significant differences of the means were measured at (5% level). Means of the cleavage rates were separated using fishers protected least significant different (LSD) test. This test was only run if there was significance difference following the ANOVA analysis.
Percoll treatment of frozen/thawed semen revealed an improved total motility greater than 70% in all three local breeds. The Boran breed had a significantly (P<0.05) higher total sperm motility (93.2±3.6) compared to Bonsmara (80.7±6.9) and Nguni (75.1±4.2) breeds. Furthermore, Boran had a significant (P<0.05) higher percentage of progressive (39.7±3.4) and rapid (36.1±5.9) motility as compared to other breeds (Table 1).
Despite some donor cows being non-responsive to flushing, the overall oocyte recovery was impressive in all breeds. Hoechst 33342 staining of developing embryo revealed clear developmental stages into the 2-4 cells to blastocyst stages of embryonic development (Figure 1). The comparison of the in vivo and in vitro embryo production methods revealed the in vivo method of embryonic development was superior in the Nguni breed at the morula stage of embryonic development than the in vitro method of embryo production (Figure 2). Also, for the Bonsmara breed, the in vivo embryo production method was superior at both the morula and blastocyst stages of embryonic development (Figure 3). In the Boran breed, the in vitro method of embryo production was significantly superior to the in vivo embryo production method at the 8-cell stage of development; however the in vivo method became superior at the blastocyst stage of development (Figure 4). Interestingly, only the Boran breed revealed a linear progression of embryonic development when the using the in vivo embryonic development method (Figure 4). The higher sperm motility observed in the Boran breed cannot account for this positive interaction between the embryo production method and the breed, since it was not observed in the in vitro method of embryo production (Table 1 and Figure 4).
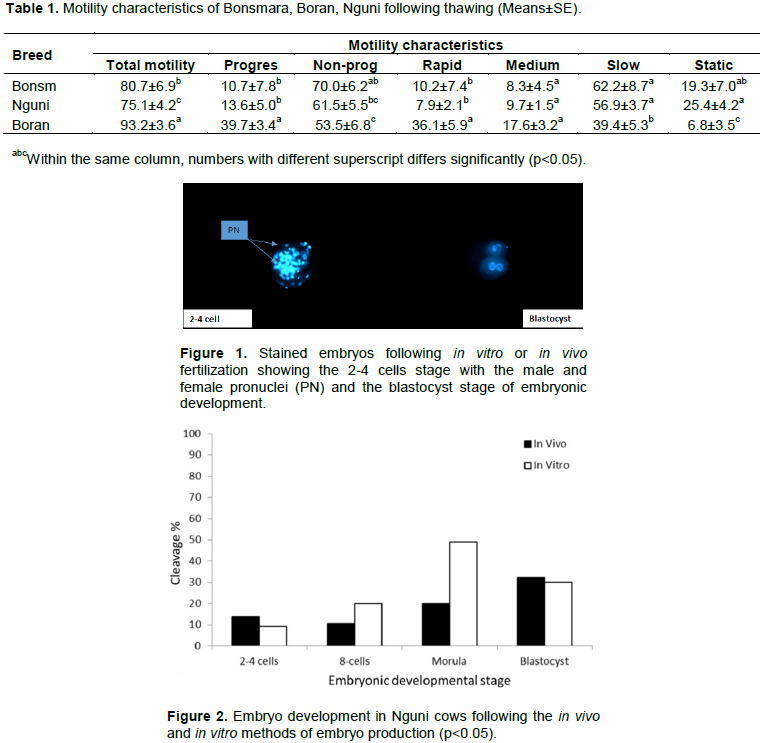
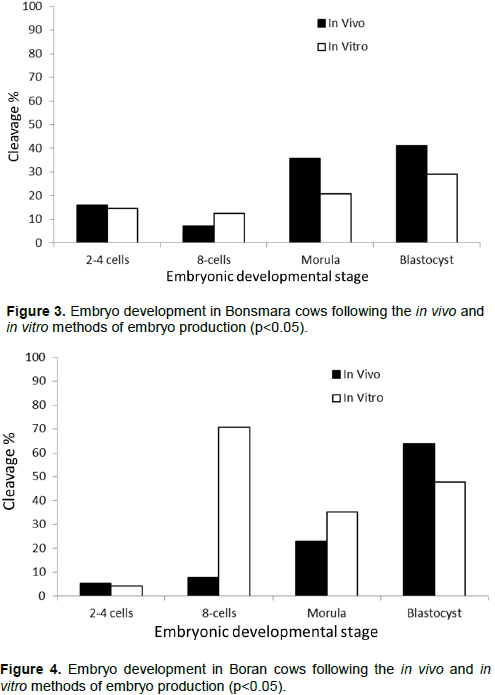
A comparison of the breed effect on the embryonic developmental stages in embryos produced by either the in vivo or in vitro method revealed that the number of 2-4 cells of the embryonic development was very low with no significant deference among all the breeds (Figure 5). At the 8-cells stage, the number of produced 8-cells of embryonic developmental stage, only embryos produced in the Boran breed revealed a highly significant number albeit under those embryos produced using the in vitro method of embryo production (Figure 6). At the morula stage of embryonic development, the Boran and Nguni breeds revealed higher number of developing embryos produced under the in vitro method, while the in vivo method produced higher number of morula stage embryos in the Bonsmara breeds (Figure 7). For the blastocyst stage of embryonic development, only the Boran breed showed significantly high number of blastocysts produced under both the in vivo and in vitro methods of embryo production as compared to the Nguni and Bonsmara breeds (Figure 8).
This study suggests that reproductive failures observed in the Nguni breed are likely to occur at the 2-4 cell stage of embryonic development when the in vivo embryo production method is used. However, both the Bonsmara and Boran breeds are likely to progress to the blastocyst stage of embryonic development when the in vivo method of embryo production were used. Ironically, when the in vitro method of embryo production was used, production failures are likely to occur at all stages of embryonic development in all the breeds.
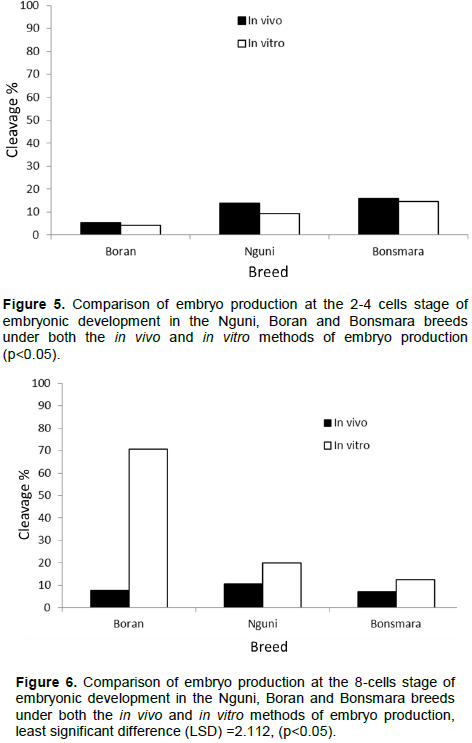
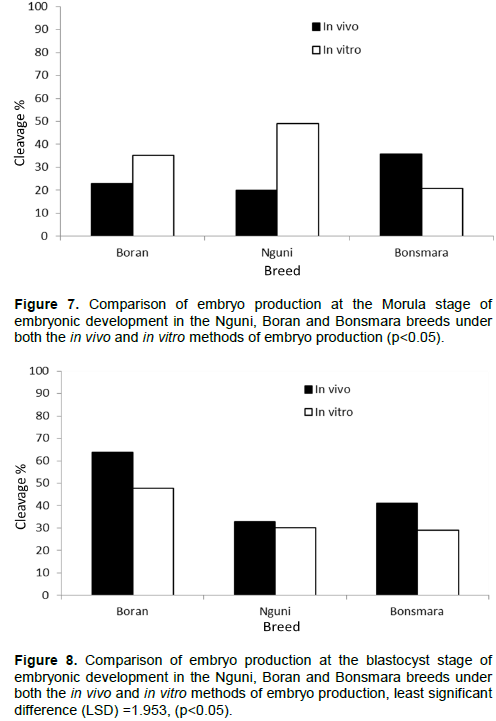
This is the first study comparing embryos produced through flushing during in vivo and in vitro fertilization in our local Nguni, Boran, and Bonsmara beef breeds. In this current study, Boran breed presented a high oocyte recovery (18.2±1.7) compared to Nguni (13±0.8) and Bonsmara (11.2±1.6) accompanied by high blastocysts produced in vivo. Moreover, the Boran breed obtained a higher number of 8-cell embryos during the in vitro production which seem to deteriorate at blastocyst stage while the opposite is true in vivo. This could suggest that the Boran breed is likely to have minimal reproductive failures as compared to Bonsmara and Nguni breeds. Overall, the development of embryos produced in vitro was low across all breeds compared to their in vivo counterparts. This study is comparable with the findings of Machatkova et al., (2008) and Lonergan and Fair (2014) who indicated that the development of embryos produced in vitro from oocytes of selected donors and survival of these embryos after cryopreservation was low compared with embryos produced in vivo from superovulated donors.
Furthermore, the study showed that semen from individual bulls may differ in their ability to fertilize oocytes to blastocyst stages after in vitro and in vivo fertilization methods. These findings are in agreement with in vitro fertilization method where semen from different bulls has been used with varying capabilities to fertilize oocytes (Abdel Dayem et al., 2009; Nagy et al., 2015). Theoretically, the higher the sperm progressive motility is accompanied by higher rates of fertilization in vitro as well as in vivo. Therefore, as previously indicated, production failures could occur since male and breed effect result in variable in vivo and in vitro outcomes in farm animals as previously discussed by others (Mahmoud et al., 2013).
In conclusion, the Boran breed seems to be superior in embryonic development following in vivo and in vitro production methods. This observation indicates that the Boran breed is less likely to have reproductive failures as compared to Nguni and Bonsmara breeds but is not immune from these failures. Also, there appears to be a relationship between the sperm motility rate and fertilization rate on in vitro and in vivo embryo development in beef cattle, as demonstrated by the Boran breed. Without excluding the failures due to female reproductive factors however, reproductive failures that occur when semen from individual bulls is used during in vivo and in vitro embryo production remains an area of greater interest among researchers. In addition, we recommend that further studies on the follicular waves of these three breeds should be studied to support the oocyte recovery rates and progressive embryonic development which might help address the observed differences.
The authors have not declared any conflict of interests.
The authors would like to acknowledge the Department of Agriculture, Forestry and Fisheries (DAFF) and the Agricultural Research Council for combined funding. The authors also acknowledge the contributions of Ms Tanja Coetzee from Embryo Plus, Professor Ngoni Moyo for valuable inputs and Professor Khoboso Lehloenya for her knowledge and advice.
REFERENCES
|
Abdel Dayem AMH, Mahmoud KGhM, Nawito MF, Ayoub MM, Scholkamy TH (2009). Fertility evaluation in Egyptian buffalo bulls using zona pellucida binding and in vitro fertilization assays. Livestock Science 122:193-198.
Crossref
|
|
|
|
Arias ME, Andara K, Briones E, Felmer R (2017). Bovine sperm separation by Swim-up and density gradients (Percoll and BoviPure): Effect on sperm quality, function and gene expression. Reproductive Biology 17:126-132.
Crossref
|
|
|
|
|
Dalton JC (2011). Semen quality factors associated with fertility. Proceedings Applied Reproductive Strategies in Beef Cattle- Northwest. Boise, ID, pp. 265-281.
|
|
|
|
|
Ferraz JBS, Eler JP, Rezende FM (2012). Impact of using artificial insemination on the multiplication of high genetic merit beef cattle in Brazil. Animal Reproduction 9(3):133-138.
|
|
|
|
|
Hossaini SM, Hajian M, Asgari V, Forozanfar M, Abedi P, Nasr Esfahani MH (2007). Novel approach of differential staining to detect necrotic cells in preimplantation embryos. Iranian Journal of Fertility and Sterility 1(3):103-106.
|
|
|
|
|
Huang SZ, Huang Y, Chen MJ, Zeng FY, Ren ZR, Zeng YT (2001). Selection of in vitro produced, transgenic embryos by nested-PCR for efficient production of transgenic goats. Theriogenology 56:545-556.
Crossref
|
|
|
|
|
Lonergan P, Fair T (2014). The ART of studying early embryo development: progress and challenges in ruminant embryo culture. Theriogenology 81:49-55.
Crossref
|
|
|
|
|
Machatkova M, Hulinska P, Reckova Z, Hanzalova K, Spanihelova J, Pospisil R (2008). In vitro production of embryos from high performance cows and the development of frozen-thawed embryos after transfer: a field study. Veterinarni Medicina 53(7):358-364
Crossref
|
|
|
|
|
Mahmoud KGM, El-Sokary AAE, Abou El-Roos MEA, Abdel Ghaffar AD, Nawito M (2013). Sperm Characteristics in Cryopreserved Buffalo Bull Semen and Field Fertility. Iranian Journal of Applied Animal Science 3(4):777-783.
|
|
|
|
|
McKenna DR, Roebert DL, Bates PK, Schmidt TB, Hale DS, Griffin DB, Savell JW, Brooks JC, Morgan B, Montgomery T, Belk K, Smith GC (2002). National beef quality audit-2000: Survey of targeted cattle and carcass characteristics related to quality, quantity, and value of fed steers and heifers. Journal of Animal Science 80:1212-1222.
Crossref
|
|
|
|
|
Mokantla E, McCrindle CM, Sebei, JP, Owen R (2004). An investigation into the causes of low calving percentage in communally grazed cattle in Jericho, North West Province. Journal of the South African Veterinary Association 75(1):30-36.
Crossref
|
|
|
|
|
Morgan JB, Savell JW, Hale DS, Miller RK, Griffin DB, Cross HR, Shackelford SD (1991). National beef tenderness survey. Journal of Animal Science 69:3274-3283.
Crossref
|
|
|
|
|
Nagy A, Polichronopoulos T, Gaspardy A, Solti L, Cseh S (2015). Correlation between bull fertility and sperm cell velocity parameters generated by computer-assisted semen analysis. Acta Veterinaria Hungarica 63(3):370-381.
Crossref
|
|
|
|
|
Nazem M, Moghadam MF, Akbari G, Eslampour MA (2016). Effect of embryo holding on bovine oocyte maturation outside the incubator. Biosciences Biotechnology Research Asia 13(3):1639-1643.
Crossref
|
|
|
|
|
Oliveira LZ, Arruda, RP, Celeghini EC, de Andrade AF, Perini AP, Resende MV, Miguel MC, Lucio AC, Hossepian de Lima VF (2012). Effects of discontinuous Percoll gradient centrifugation on the quality of bovine spermatozoa evaluated with computer-assisted semen analysis and fluorescent probes association. Andrologia 44(1):9-15.
Crossref
|
|
|
|
|
Petyim S, Bage R, Forsberg M, Rodriguez-Martinez H, Larsson B (2000). The effect of repeated follicular puncture on ovarian function in dairy heifers. Journal of Veterinary Medicine. Series A: Physiology, Pathology, Clinical Medicine 47:627-640.
Crossref
|
|
|
|
|
Pontes JHF, Nonato-Junior I, Sanches BV, Ereno-Junior JC, Uvo S, Barreiros TRR, Oliveira JA, Hasler JF, Seneda MM (2009). Comparison of embryo yield and pregnancy rate between in vivo and in vitro methods in the same Nelore (Bos indicus) donor cows. Theriogenology 71(4):690-697.
Crossref
|
|
|
|
|
Scholtz MM (1988). Selection possibilities of hardy beef breeds in Africa: The Nguni example. In Proceedings of the 3rd world congress on sheep and beef cattle breeds, Paris, France pp. 303-319.
|
|
|
|
|
Scholtz MM, Theunissen A (2010). The use of indigenous cattle in terminal crossbreeding to improve beef cattle production in Sub-Saharan Africa. Animal Genetic Resources Information 46:33-36.
Crossref
|
|
|
|
|
Strydom PE, Frylinck L, Van der Westhuizen J, Burrow HM (2008). Growth performance, feed efficiency and carcass and meat quality of tropically adapted breed types from different farming systems in South Africa. Australian Journal of Experimental Agriculture 48:599-607.
Crossref
|
|
|
|
|
Sudano MJ, Paschoa DM, Maziero RRD, Rascado TS, Guastali MD, Crocomo LF, Magalhães LCO, Monteiro BA, Martins A, Machado R, Landim-Alvarenga FDC (2013). Improving post-cryopreservation survival capacity: an embryo-focused approach. Animal Reproduction 10:160-167.
|
|
|
|
|
Watson PF (2000). The causes of reduced fertility with cryopreserved semen. Animal Reproduction Science 60-61:481-492.
Crossref
|
|