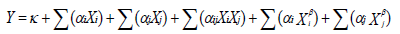ABSTRACT
The present study aims at optimizing the production of Saccharomyces cerevisiae SKM10 single cell proteins (SCP) from mango waste. To optimize the production process, the effect of two independent variables was evaluated by a three-level full factorial design using response surface methodology (RSM). The parameters studied were inoculum size (2 to 12% v/v) and concentration of substrates (5 to 10 g/L). Analytical methods were used to determine yeast cell biomass, sugar and protein content in yeast. Under optimal conditions, sugar content and maximum cell dry weight attained were 15.28 and 29.85% (w/w). Optimal protein content of 79.14% (w/w) was achieved at 8% (v/v) inoculum and 8% (g/L) substrate concentration. These results have provided useful information on how to improve the production by RSM and suggested that S. cerevisiae SKM10 might be applied effectively to produce SCP using mango waste as a low-cost substrate.
Key words: Mango waste, Saccharomyces cerevisiae, bioconversion, production, single cell protein.
Burkina Faso and other developing countries have long had issues of processing and preserving locally produced agricultural food products. Agriculture and the agri-food industries release important by-products generally considered as source of pollution (straw, vegetable residues, agri-industries residues). Therefore, the recuperation and valorization of these by-products to food constitute an interesting alternative. This is concerning the production of single cell protein (Manilal et al., 1991; Hussain et al., 1992). In Burkina Faso, lack of solutions to these problems led to enormous losses of the fruits harvest. Five thousand tons of mango (Magnifera indica L.) are lost per year in Burkina Faso. The possibilities of energizing valorization of the biomass are offered by the agricultural products of weak commercial value as agri-food industries and residues of fruits. So mango residues coming from industrial area, market and site stockage can attain 50000 tons per year. These residues generate annual environmental pollution (Somda et al., 2010, 2011). During the processing of mango, by-products such as its peel and kernel are generated. Peel contributes about 15 to 20% of the fruit. Since peel is not currently utilized for any commercial purposes, it is discarded as a waste and becoming a source of pollution. This waste should be treated as specialized residue due to its high content of carbohydrates (Somda et al., 2011). Mango contains tannins, carbohydrates as starch, pectins, cellulose, fructose with significant concentration of glucose (Ajila et al., 2007). Due to its important carbohydrates rate, mango can be a valuable fermented substrate for both single cell protein (SCP) and metabolites production using Saccharomyces cerevisiae.
Many microbial products can be developed from their microbial biomass because of their rich composition: Carbohydrates, lipids, proteins, nucleic acids, vitamins, etc. In addition, their SCP contains microorganisms involved in the conversion of carbohydrates and related components to end products such as acids, alcohols and carbon dioxide (Bamforth, 2005).
In recent years, increasing attention has been given to the conversion of food processing wastes into valuable by-products such as the production of yeast protein from wastes. SCP is obtained by the fermentation of microorganisms on an organic substrate (Ghaly et al., 1992, 1993). It has long been recognized as a digestible and useful food resource through the production of SCP. The richness of essential amino acid makes it to be approved as food additives (Rosma et al., 2005).
The interest of these micro-organisms resides in the fact that it contains amino acids. SCP can be obtained by culturing of bacteria, yeasts, molds and algae (Ceccato et al., 1992).
Yeast is mostly used for human feeding because it has a high product yield, extended stable products with little risk of bacterial contamination (Barrios-Gonzalez, 2012). Due to its composition, SCP from S. cerevisiae is considered as source of food balance for human or animal nutrition. SCP products are very rich in protein with a wide spectrum of amino acids and vitamins, but low concentrations of fat and are cholesterol-free. These show their potential as suitable candidates for many versatile applications (Nasseri et al., 2011). Amino acids of SCP are critical to life and are used as food or feed additives, in parenteral nutrition or as building blocks protein (Wendisch et al., 2016).
Yeast extract (YE) has been reported as a good source of supplement for protein deficient diet. Protein content from dried yeast biomass may range from 45 to 50% (w/w) and over 60% w/w is in YE, thus making it an important SCP source (Sgarbieri et al., 1999). It has been recognized as food resource and approved as food additive by the United States Food and Drug Administration (FDA) (Rosma et al., 2005). This study aims to valorize by-products of mango residues by optimizing production of SCP using response surface methodology (RSM).
Strain and inoculation preparation
S. cerevisiae SKM10 previously isolated from fermented food was obtained from the culture collection of Laboratory of Microbiology and Microbial Biotechnology, Department of Biochemistry and Microbiology, University Ouaga1 Pr Joseph KI-ZERBO, Burkina Faso. The yeast isolates were streaked on the surface of yeast extract, peptone, and glucose (YEPG) agar slants (Biomerieux) at 4°C.
Preparation of mango residue
Mango residue was collected from waste dumping sites (Banfora, Bobo-Dioulasso, Ouagadougou) and treated using the process of Somda et al. (2011). The extract was obtained and the suspension centrifuged (10.000 rpm for 15 min) to remove the supernatant residue. Also, the extract was diluted, and filtered using filter paper (Whatman No. 1, Sigma-Aldrich). It was pasteurized twice for 3 min at 60°C after pH was adjusted to 4.0 with citrate buffer (Somda et al., 2010).
Culture and fermentation conditions
The preparation of inoculum was carried out in an orbital shaker at 150 rpm in 250 mL triangular flasks containing each 100 mL of growth medium (nutrients broth). The process was monitored at 30°C for 18 h to obtain 105 cells/ml inoculum. For the fermentation, flasks containing 2 L of mango waste medium were inoculated with a cell suspension at 2 to 12% (v/v). The mixture was cultivated at 30°C and 200 rpm for 30 h. The final fermentation medium contained 5 to 10 g.L-1 of substrate (Somda et al., 2011).
Biomass and extraction of protein from yeast cells
After 24 h, cells were harvested by centrifugation at 20000 rpm for 20 min (Olsson and Nielsen, 1997); they were washed twice with distilled water and dried in an oven at 50°C for 48 h and weighed.
The dried cells were mixed with glass beads of 0.45 to 0.50 mm in diameter and acetate buffer (pH 5.0, 4°C) at a ratio of 1:4:4 (w:w:v), respectively. Mechanical rupturing of yeast cells was achieved through vortexing of the mixture for 30 s and stored in ice-bath for 1 min. After centrifugation, crude protein was freeze-dried (-50°C, vacuum 100 g, 24 h) and stored for analysis (Catley, 1988).
Analytical process
The concentration of yeast cells in the fermenting mash was measured using turbidimetric (absorbance at 600 nm) method and by determining dry weight of yeast cells (Lagzouli et al., 2007). Dry cell weight was determined gravimetrically by the method of Olsson and Nielsen (1997). Nitrogen content of yeast biomass was determined by micro-Kjeldhal method. The crude protein values were obtained by multiplying the nitrogen content by 6.25 (Lopez et al., 2010). Total sugar was analyzed using the procedure mentioned in AOAC adapted methods (AOAC, 2016). Reducing sugars was determined colorimetrically using a dinitrosalicylic (DNS) acid reagent (Miller, 1959).
Design of the experiment using RSM
A 23 factorial design was implemented in order to evaluate the optimum operational conditions for the batch fermentation of S. cerevisiae SKM10 using mango residue medium. The dependant variables chosen were protein content in yeast, yeast growth and sugar content. The independent variables used for this study were inoculum size and concentration of initial sugar substrate in the medium. The units and the coded levels of the independent variables are shown in Table 1. Experimental data are mean of triplicate determination.
Statistical analysis
Statistical analysis was carried out using the Design Expert version 10.0.6 software (Stat-Ease Corp., Minneapolis). A three-level full factorial design was used to develop a quantitative interpretation of mathematical models between the two variables studied: rate of inoculum [2.0 to 12.0% (v/v)] and concentration of substrate [5 to 10 (g/L)]. Analysis of variance (ANOVA) was performed and the significance of the model was examined with Fisher’s statistical test (F-test) used for the significant difference between the sources of variation in experimental results, significance of regression, and coefficient of multiple determination (R2).
Optimization of parameters by RSM
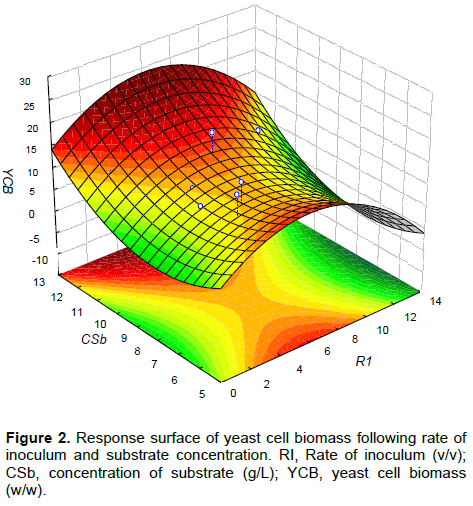
The 3-level full factorial experimental design matrix and the results obtained for all dependent variables are shown in Table 2. These results were used to develop RSMs based on the following equation:
Where, Y (n:1-3) is the dependent variable, Xi and Xj are the independent variables, β: exposant, αi and αj are the coefficients obtained by multiple regression of the experimental data.
The analysis of the experimental data was carried out using the RSM for optimization of experimental parameters. The different equations generated are shown in Table 3.
The ANOVA of the quadratic model is shown in Table 4. The models F-value showed 10.78, 15.20 and 13.89, respectively for protein content, sugar content and yeast cell biomass as the dependent variables. R2 values were found respectively as 0.88, 0.99 and 0.95.
Regression analysis was used to identify critical components with significant p value (<0.05) influencing the production. Their different models P-value (Prob>F) ranged at significant level (P<0.05). These corresponding P-values suggest that, dependent variables used in the study are significant model terms. The production of yeast cell biomass (P=0.0013) was the largest influenced by rate of inoculum and substrate concentration, followed by sugar content of yeast (P=0.025) and protein content (P=0.043).
Production of sugar content in yeast
The analysis of response surface indicated that sugar content of yeast ranged from 15.6 to 29.85% (w/w) as shown in Figure 1. The highest sugar content (29.85% (w/w)) was produced at 7% (v/v) of inoculum and 8% (g/L) of substrate concentration. However, sugar content decreased under optimal condition.
Efficiency of sugar production was strongly correlated (R2=0.025) to influence both factors: inoculum and nature and concentration of substrate. This correlation was confirmed by ANOVA test which revealed high value of R2 (0.88) mentioned in Table 4. The predicted optimal value, 29.85% (w/w) has demonstrated an increase of 47.74% considering the initial condition (Inoculum 2% and substrate 5%). Maximal sugar content in this study was higher compared to that of Rosma et al. (2005) found on yeast cell [27.17% (w/w)] and lower than those obtained (40.7 and 32.77% (w/w)), respectively by Forster et al. (2003) and Verduyn (1991). The difference of results in this experiment with other authors could be explained with the nature of substrate, the strain genetic background and the oxygenation degree (Carnicer et al., 2009).
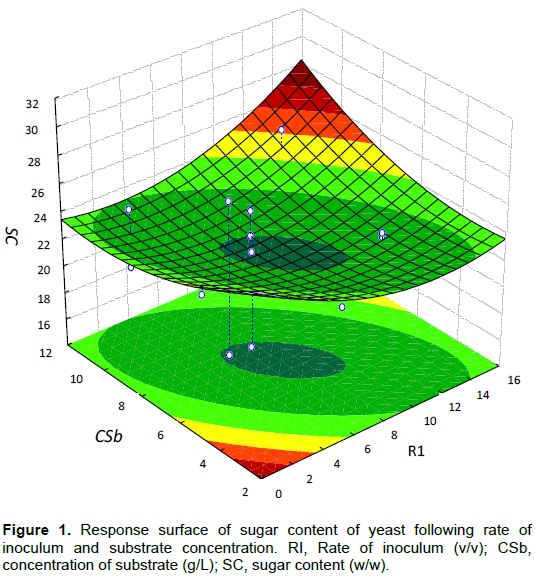
Production of yeast cell biomass
The 3D response surface graph showed that yeast cell biomass varied from 5.93 to 15.28 (g/L). The predicted maximum value of production was 15.28% and increased at 61.20% compared to the non-optimized condition (5 g/L of substrate). However, substrate of more than 8 g/L was found to have adverse effect and resulted in lesser biomass yield. The biomass maximal amount obtained is similar to Rosma et al. (2005)’s results (15.25 g/L) and it is significantly higher than values found by Somda et al. (2010) for Saccharomyces species (8.92 g/L) and also by Gao et al. (2012) and Ouedraogo et al. (2017) who obtained 10.83 and 8.29 g/L, respectively from Candida tropicalis and Candida utilis NOY1. P-value (0.013) exhibited significant influence of independent factors on biomass increase (Figure 2).
The high R2 value (0.95) indicated that the model equation can adequately predict the response. The 3D plot showed that the interactions among the independent variables signiï¬cantly influenced the biomass production, indicating the increase of the predicted response (Hu et al., 2016). The nature and concentration of sugar in the medium play a vital role in the improvement of fermentation efficiency (Nancib et al., 2001), but the biomass production is particularly based on size and species of inoculum (Somda et al., 2010).
Production of protein
Using the RSM for optimization of production, the protein content obtained ranged from 45.58 to 79.14% (w/w) as shown in Figure 3. From the combinations of effects of independent variables on the protein production (Table 4), it can be noted that the optimal production (79.14% (w/w)) was achieved at 8% (v/v) inoculum and 8% (g/L) substrate concentration. An increase of 42.41% was noted in comparison with non-optimized condition.
For the purpose of validating the models, the experiment was performed under the optimal conditions. Hence, rate of inoculum and substrate concentration exhibited significant influence concerning protein content (p=0.043). The protein content in this study was higher compared to the results of Sgarbieri et al. (1999), Roma et al. (2005), Gao et al. (2012) and Ouedraogo et al. (2017) who found 61.5, 66.61, 56.42 and 54.80%, respectively (w/w).
This remark could be explained by the composition of substrate, strain enzymatic capacity and degree of process optimization. So, the composition of substrate could contribute to improve the efficiency of the process by increasing the availability of reducing sugar to yeast (Somda et al., 2011a). Mango was detected to be the most preferred substrate by yeast for growth and a concentration of 8 g/L (total sugar) was found to be optimal for S. cerevisiae. It allows high cell viability and protein production. Coman et al. (2012) showed that protein production was influenced by the time of cultivation and concentration of substrate.
Factorial design and RSM have been proved to be effective in optimizing production of S. cerevisiae using mango waste. The significant influence of dependent variables (inoculum and substrate) on yeast production has been noted. Yeast cell biomass, sugar and protein content increase and are close to the predicted values. This remark demonstrated the reliability of the model tested. The results suggest that mango waste could be a valuable substrate for S. cerevisiae production and could also be an opportunity to reduce the environmental pollution caused by the agro-industrial by-products.
The authors have not declared any conflict of interests.
REFERENCES
|
Ajila CM, Bhat SG, Rao UJSP (2007). Valuable components fo raw and ripe peels from two indian mango varieies. Food Chem. 102:1006-1011.
Crossref
|
|
|
|
Association of Official Analytical Chemists (AOAC) (2016). The official methods of analysis of AOAC International, 20th edn. George W. Latimer, Jr. 3172p.
|
|
|
|
|
Bamforth CW (2005). Food, fermentation and microorganisms. Blackwell Science Ltd. Oxford. pp. 1-38.
Crossref
|
|
|
|
|
Barrios-Gonzalez J (2012). Solid-state fermentation: physiology of solid medium, its molecular basis and applications. Process Biochem. 47:175-185.
Crossref
|
|
|
|
|
Carnicer M, Baumann K, Toplitz I, Sánchez-Ferrando F, Mattanovich D, Ferrer P, Albiol J (2009). Macromolecular and elemental composition analysis and extracellular metabolite balances of Pichia pastoris growing at different oxygen levels. Microb. Cell Fact. 8(65):1-14.
Crossref
|
|
|
|
|
Catley BJ (1988). Isolation and analysis of cell walls. In: Yeast: A pratical approach (Campell I and Duffus JH, eds). Whashington: IRL Press, Oxford, Washington, USA. pp. 163-183.
|
|
|
|
|
Ceccato SR, Antonini ET, Tauk SM (1992). Amino acid composition of single and mixed fungal cultures grows in sugar cane vinasse. Rev. Microbiol. 23(1):43-47.
|
|
|
|
|
Coman G, Leustean I, Georgescu L, Bahrim G (2012). Optimization of protein production by Geotrichum candidum miug 2.15 by cultivation on paper residues, using response surface methodology. BioResources 7(4):5290-5303.
Crossref
|
|
|
|
|
Forster J, Famili I, Fu PC, Palsson BO, Nielsen J (2003). Genome-scalereconstruction of the Saccharomyces cerevisiae metabolic network. Genome Res. 13:244-253.
Crossref
|
|
|
|
|
Gao Y, Li D, Liu Y (2012). Production of single cell protein from soy molasses using Candida tropicalis. Ann. Microbiol. 62(3):1165-1172.
Crossref
|
|
|
|
|
Ghaly AE, Ben-Hassane RM, Mansour MH (1992). Effect of pH Control on the Growth and Survival of Kluyveromyces fragilis in Cheese whey under Aerobic Condition. Appl. Biochem. Biotechnol. 33(91):3303-0219.
|
|
|
|
|
Ghaly AE, Ben-Hassane RM, Mansour MH, Nassar MA (1993). Modelling batch production of single cell protein from cheese whey. Appl. Biochem. Biotechnol. 43(1):25-36.
Crossref
|
|
|
|
|
Hu Y, Qin H, Zhan Z, Dun Y, Zhou Y, Peng N, Ling H, Liang Y, Zhao S (2016). Optimization of Saccharomyces boulardii production in solid-state fermentation with response surface methodology. Biotechnol. Biotechnol. Equip. 30:(1)173-179.
Crossref
|
|
|
|
|
Hussain AM, El Saied H, Yasin MH (1992). Bioconversion of Hemicelluloses of Rice Hull Black Acid into Single Cell Protein. J. Chem. Technol. Biotechnol. Egypt. 53(2):147-152.
Crossref
|
|
|
|
|
Lagzouli M, Mennane Z, Aitounejjar A, Wafae S, Ouhssine M, Elyachioui M, Berny EH, Jadal M (2007). Optimization of growth and extracellular glucoamylase production by Candida famata isolate. Afr. J. Biotechnol. 6(22):2590-2595.
Crossref
|
|
|
|
|
Lopez CVG, Garcia MCC, Fernandez FGA, Bustos CS, Chisti Y, Sevilla JMF (2010). Protein measurements of microalgal and cyanobacterial biomass. Bioresour. Technol. 101:7587-7591.
Crossref
|
|
|
|
|
Manilal VB, Narayanan CS, Balagopolam C (1991). Cassava starch effluent treatement with concomitent SCP Production. World J. Microbiol. Biotechnol. 7(2):185-190.
Crossref
|
|
|
|
|
Miller GL (1958). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Biochem. 31(3):426-428.
|
|
|
|
|
Nancib N, Nancib A, Boudjelal A, Benslimane C, Blanchard F, Boudrant J (2001). The effect of supplementation by different nitrogen sources on the production of lactic acid from date juice by Lactobacillus casei subsp. Rhamnosus. Bioresour. Technol. 78:149-153.
Crossref
|
|
|
|
|
Nasseri AT, Rasoul-amini S, Moromvat MH, Ghasemi Y (2011). Single cell protein: Production and process. Am. J. Food Technol. 6(2):103-116.
Crossref
|
|
|
|
|
Olsson L, Nielsen J (1997). Online and in situ monitoring of biomass in submerged cultivations. Trends Biotechnol. 15(12):517-522.
Crossref
|
|
|
|
|
Ouedraogo N, Savadogo A, Somda MK, Tapsoba F, Zongo C, Traore AS (2017). Effect of mineral salts and nitrogen source on yeast (Candida utilis NOY1) biomass production using tubers wastes. Afr. J. Biotechnol. 16(8):359-365.
Crossref
|
|
|
|
|
Rosma A, Liong MT, Mohd Azemi MN, Wan Nadiah WA (2005). Optimization of Single cell protein production by Candida utilis using juice extracted from pineapple waste through Response Surface Methodology. Malays. J. Microbiol. 1(1):18-24.
|
|
|
|
|
Sgarbieri VC, Alvim ID, Vilela ESD, Baldidni BLS, Bragagnolo N(1999). Pilot plan production of yeast Saccharomyces cerevisiae derivatives for use as ingredient in food formulations. Braz. J. Food Technol. 2(2):119-125.
|
|
|
|
|
Somda MK, Savadogo A, Ouattara CAT, Ouattara AS, Traoré AS (2010). Production of alcohol from mango (Mangifera Indica L.) using strains of Saccharomyces and Schizosaccharomyces genera isolated from wasted mangos in Burkina Faso. Biosci. Biotechnol. Res. Asia 7(2):529-536.
|
|
|
|
|
Somda MK, Savadogo A, Ouattara CAT, Ouattara AS, Traoré AS (2011). Improvement of bioethanol production using amylasic properties from Bacillus licheniformis and yeast strains fermentation for biomass valorization. Asian J. Biotechnol. 3(3):254-261.
Crossref
|
|
|
|
|
Verduyn C (1991). Physiology of yeasts in relation to biomass yields. Antonie van Leeuwenhoek. 60(3-4):325-353.
Crossref
|
|
|
|
|
Wendisch FV, Jorge JMP, Perez-Garcıa F, Sgobba E (2016). Updates on industrial production of amino acids using Corynebacterium glutamicum. World J. Microbiol. Biotechnol. 32:105-115.
Crossref
|
|