ABSTRACT
Tannase (tannin acyl hydrolse, E.C.3.1.1.20) is an enzyme that catalyzes the hydrolysis of ester and depside bonds of hydrolysable tannins. Among the filamentous fungi the genus Penicillium is recognized as the second best producer of tannase. This enzyme presents applications in several industrial segments, such as in the production of judges and teas. The industrial production of tannase can be performed through solid state fermentation (SSF) by the use of tannin rich fruit residues, in order to minimize production costs. In the present study 31 species of Penicillium isolated from Caatinga and Atlantic Forest were tested quantitatively for the production of tannase. The species were kept in the Collection of Cultures Micoteca - URM (WDCM604) under mineral oil. The enzyme production was carried out under SSF using “cajá” (Spondias lutea L.) and Manga (Mangifera indica L.) residues that are rich in tannins. Mango residue proved to be the best inducer of the production of tannase by Penicillium species, most of which presented high tannase activity, ranging from 14.48 to 89.48 U mL-1. The three best producers were Penicillium rolfssi URM6216 with 89.49 U mL-1, Penicillium janthinellum URM5993 with 85.93 U mL-1 and Penicillium glabrum URM6092 with 59.26 U mL-1. Therefore, P. rolfssi URM6216 is being indicated for optimization of tannase production by SSF, using mango residue as a substrate. This is the first study on the potential of hog-plum (cajá) and mango wastes as substrates for tannase production by filamentous fungi.
Key words: Penicillium rolfssi URM6216, tannin acyl hydrolase, waste, mango, cajá.
Brazil is considered the country with the greatest biodiversity on the planet (Lejon et al., 2005; Reis, 2007). Due to its continental size and the great geomorphological and climatic variation, this country houses seven biomes: Amazon, Caatinga, Cerrado, Coastal, Pantanal, Atlantic Forest and Campos Sulinos. These biomes are home to
innumerable species of animals, plants and micro-organisms, especially fungi (Lejon et al., 2005; Carvalho et al., 2010).
Soil is considered to be one of the main habitats for populations of microorganisms including fungi (Singh et al., 2011). Among the fungi commonly isolated from soil are species of Penicillium. Most of these species are saprophytic, not very nutritionally demanding, giving them the ability to grow in any environment where there is a minimum of mineral salts or the most diverse and complex organic carbon sources (Pitt, 1991). This genus is of utmost relevance in nature, as its species participate actively in the recycling of organic matter (Samson and Frisvad, 2004; Cruz et al., 2013a).
In addition to their relevant environmental importance in the recycling of organic matter, Penicillium species have broad biotechnological potential, being used for the production of enzymes of industrial, environmental, pharmaceutical and food interest, among others (Bon et al., 2008). Among the various enzymes produced by Penicillium spp., tannase is worth pointing out (Belur and Mugeraya, 2011).
Tannase or tannin acylhydrolase (TAH) (EC 3.1.1.20) is an inducible enzyme that catalyzes the hydrolysis of ester links and depsides in hydrolyzable tannins, such as tannic acid, releasing glucose and gallic acid (Selwal et al., 2011; Madeira Jr. et al., 2012; Govindarajan et al., 2016). As tannins are produced by plants as a defense mechanism, these compounds can be found in all parts of the plant, from cellular vacuoles, leaves, bark, fruits and seeds to sap (Monteiro et al., 2005). Many microorganisms produce tannase as a form of counter-attack to plants (Pinto et al., 2005b).
Tannase may have wide application in the pharmaceutical, cosmetic and beverage industries, mainly in the production of beer. Another important application of tannase occurs in the manufacture of juices whose fruits have high tannin content. This enzyme is still applied for the production of gallic acid in the color stabilization of wines and coffee-based soft drinks, in the treatment process of leather, food detannification and effluent treatment in the leather industry (Madeira Jr. et al., 2012; Govindarajan et al., 2016). Tannase-producing soil microorganisms play an active role in the decomposition and recycling of plant materials rich in tannins and avoid environmental pollution problems (Battestin et al., 2004).
Among the filamentous fungi, Aspergillus is considered the genus that best produces tannase, followed by the genus Penicillium, whether by means of submerged fermentation (SmF) or solid state fermentation (SSF) (Aguilar et al., 2007; Renovato et al., 2011; Chávez-González et al., 2012; Costa et al., 2012)). However, although the potential has been proven of species of the genus Penicillium to produce tannase, studies are still scarce.
Due to the high importance of application of microbial tannase in various industries, it is necessary to search for production processes that will minimize costs, increase production and contribute directly to environmental balance, through the utilization of agro-industrial wastes that are commonly disposed of in the environment without previous treatment. In this context, SSF presents itself as an excellent alternative for tannase production by fungi (Lima et al., 2014).
Solid state fermentation is a process in which a microorganism grows in solid of damp substrates, usually vegetable waste, in the absence of free water (Pandey, 2003; Lima et al., 2014; Madeira Jr. et al., 2012). Agribusiness and food industries produce large amounts of waste, both liquid and solid. These residues may present significant challenges for final disposal and have high polluting potential, in addition to often representing losses of biomass and nutrients of high value, as is the case of wastes generated by industries producing fruit pulps, such as residues of hog-plum (Spondias lutea L.) and mango (Mangifera indica L.).
It is estimated that annually in Brazil, both for hog-plum and for mango, about 35 tons of waste are produced by the fruit pulp industry. Such waste, as it contains a high tannin content, can be a viable alternative for tannase production by SSF using fungi (Pinto et al., Pinto, 2016; Chávez-González et al., 2012).
Given the above, the objectives of the present work were to evaluate Penicillium species isolated from soils regarding the production of tannase through SSF using hog-plum (Spondias lutea L.) and mango (Mangifera indica L.) wastes generated by the fruit pulp industry in Pernambuco, Brazil.
Micro-organisms
The microorganisms used in the present study were isolated from soil in areas of the Atlantic Forest and Caatinga biomes and were kept in the Collection of Cultures Micoteca - URM (WDCM604), from the Center of Biosciences of the Federal University of Pernambuco, Brazil.
Detection of tannolytic capacity of species of Penicillium
31 isolates of different species of Penicillium were tested for the ability to degrade tannic acid. Each species of Penicillium was transferred to the center of a Petri dish containing Czapek agar medium (C12H22O11, 30 g; MgSO4.7H2O, 0.5 g; KCl, 0.5 g; FeSO4.7H2O, 0.01 g; KH2PO4, 1.0 g; NaNO3, 3.0 g; agar, 16 g; distilled H2O, 1000 mL) modified by substituting sucrose for 5 g L-1 of tannic acid (C76H42O56). The tannic acid was sterilized separately by means of filtering through a 0.22 µm membrane. Each species was streaked in triplicate and incubated in an oven at 30°C for 72 h (Murugan et al., 2007).
Production of tannase under SSF
Origin of wastes
Wastes of hog-plum (Spondias lutea L.) and mango (Mangifera indica L.) were obtained from the fruit pulp industry in Recife, PE, Brazil. The waste was flushed with sterile distilled water and dried in an oven at 55°C for 48 h.
Tannin estimation
The estimation of tannin content was done following the protein precipitation method (Hagerman and Butler, 1981). Wastes of hog-plum (Spondias lutea L.) and mango (Mangifera indica L.) were ground into particles of 50 µm in methanol and kept overnight at 4°C. One milliliter of extract was taken in a tube and 3 mL of BSA solution was added and kept for 15 min at room temperature. The tubes were centrifuged at 5000 g for 10 min, supernatant was discarded, and pellet was dissolved in 3 mL of SDS-triethanolamine solution. One milliliter of FeCl3 solution was added and tubes were kept for 15 min at room temperature for color stabilization. Color was read at 530 nm against the blank.
Inoculum preparation
The strains was grown and maintained on malt extract agar (MEA) slants by culturing at 30ºC. Cultures were preserved at 4ºC for short-term storage. To a fully sporulated, 1-week-old, agar slant culture, 10 mL of sterile distilled water with 0.1% Tween-80 was added. The spores were scraped using an inoculation needle under strict aseptic conditions. The spore suspension thus obtained was used as the inoculum. Viable spores in the spore suspension were determined by the plate count technique (Sabu et al., 2005).
Moistening medium and preparation of SSF medium for inoculation
A salt solution with 0.5% w/v NH4NO3, 0.1% w/v MgSO4.7H2O and 0.1% w/v NaCl was used as the moistening medium for SSF. Final pH of the medium was adjusted to 5.0 (Sabu et al., 2005).
Five grams of hog-plum and mango residue were separately added to 250 mL Erlenmeyer flasks, moistened with 5 mL of salt solution, autoclaved at 121ºC for 20 min, cooled to room temperature and inoculated with 1 mL of the fungal spore inoculum (5×108 spores/mL). The contents were mixed thoroughly and incubated at 30ºC for 96 h (Sabu et al., 2005).
Enzyme extraction
The fermented substrates were mixed thoroughly by keeping the flasks on a rotary shaker (Scigenics, India) at 150 rpm for 10 min after adding 50 ml of distilled water with 0.01% Tween 80. Crude enzyme from the fermented matter was extracted by direct filtration using Whatman #1 filter paper. The filtrate was collected in vials and preserved at 4ºC for further analysis (Sabu et al., 2005).
Enzyme assay
Tannase activity was estimated by the method of Sharma et al. (2000). The method is based on the formation of a chromogen between gallic acid (released by the action of tannase on methyl gallate) and rhodanine (2-thio-4-ketothiazolidine). The pink color developed was read at 520 nm using a spectrophotometer (Hitachi-U5100). Tannase activity was expressed in international units. One unit of tannase activity was defined as the amount of enzyme required to liberate one micromole of gallic acid per minute under the defined reaction conditions.
The mango waste (Mangifera indica L.) presented higher tannin content (15.25 U/g), followed by the residue of waste hog-plum (Spondias lutea L.) (11.3 U/g). Of the 31 species isolated, identified and tested for tannolytic ability in solid medium only Penicillium janthinellum, P. lanosum and P. mellini, did not produce a degradation halo (Table 1). Penicillium lapidosum did not grow in the presence of tannic acid. Due to the low number of cultures that did not present a qualitative capacity for tannase production, all species were selected for quantitative evaluation by SSF from the use of hog-plum (Spondias lutea L.) and mango (Mangifera indica L.) waste resulting from the fruit pulp industry.
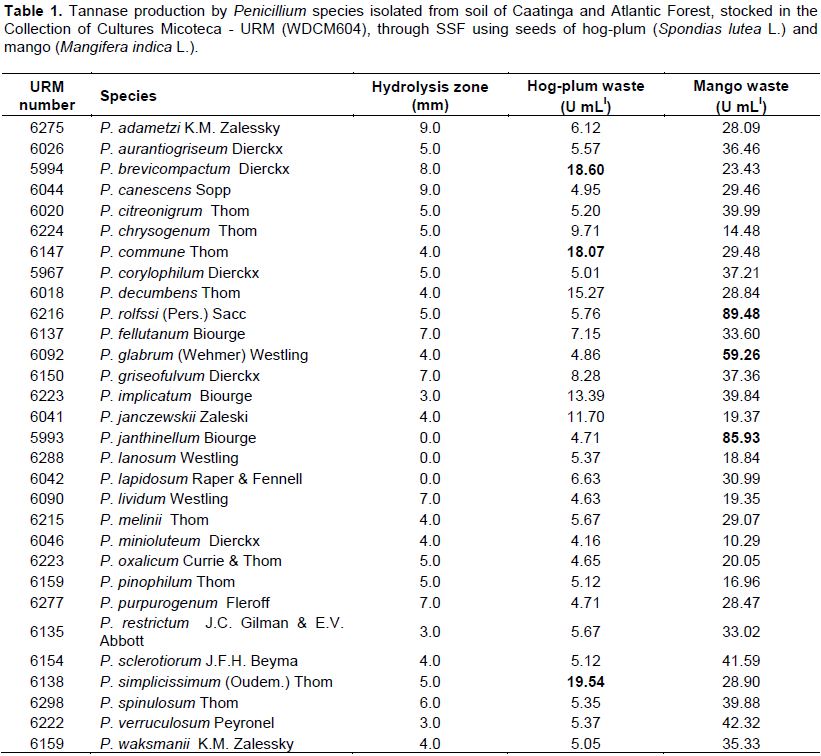
By means of SSF, all species analyzed produced tannase in the two residues tested (Table 1). Penicillium adametzi, P. aurantiogriseum, P. brevicompactum, P. citreonigrum, P. decumbens, P. fellutanum, P. janczewskii, P. janthinellum, P. lividum, P. melinii, P. minioluteum, P. pinophilum, P. sclerotiorum, P. simplicissimum, P. spinulosum, P. verruculosum and P. waksmanii, for the first time, are being reported as producers of this enzyme (Table 1).
Tannase production varied according to the microorganism and residue evaluated, as the residue is an important stimulant for the microorganism to produce the enzyme. The mango residue proved to be the best inducer of tannase production by Penicillium species, most species presented high tannase activity, ranging from 14.48 to 89.48 U mL-l. The top three producers were Penicillium rolfssi URM6216 with 89.49 U mL-l, P. janthinellum URM5993 with 85.93 U mL-l and P. glabrum URM6092 with 59.26 U mL-l. In the hog-plum residue, the least activity was 4.16 U mL-l and the maximum, 18.60 U mL-l, with the top three producers being P. simplicissimum URM6138, P. brevicompactum URM5994 and P. commune URM6147, with 19.54, 18.60 and 18.07 U mL-l, respectively (Table 1). Therefore, P. rolfssi URM6216 is indicated for the optimization of tannase production through SSF using mango residue as a substrate.
Tannase was discovered accidentally by Tieghem in 1967, in an experiment aimed at creating gallic acid in an aqueous solution of tannins, where two species of filamentous fungi were growing, later identified as Penicillium glaucum and Aspergillus niger (Aguilar et al., 2007). Later, various microorganisms have been reported as producers of tannase: bacteria, yeasts and filamentous fungi.
Although the potential of species of the genus Penicillium to produce tannase has been proven, studies are still scarce. In a bibliographic survey conducted by Aguilar et al. (2007), between the years 1969 to 2007, only five works are cited. A similar survey was conducted in 2011 by Belur and Mugeraya. The authors stated that there was no change in the number of works on production of tannase by Penicillium species from 2007 to 2011.
Solid state fermentation for the production of enzymes offers advantages over the conventional method of submerged fermentation (Pinto et al., 2005a; Cruz et al., 2013b). The means of production is simple: Agro-industrial by-products can be used, among the most common of which are grape skins, cashew fruit residue, coffee bean residue and bran of wheat, rice and oats, together with tannic acid (Lima et al., 2014). However, some fruits rich in tannins and grown in Brazil, have not yet been studied for the production of tannase, among them hog-plum (Spondias lutea L.) and mango (Mangifera indica L.).
Hog-plum, a fruit originating from tropical America, belongs to the family Anacardiaceae. It has been widely accepted in the market, being considered quite tasty and nutritious (Bosco et al., 2000; Gois et al., 2014). In addition to its regional importance, hog-plum has been gaining a prominent role in Brazilian agribusiness, with the development of new products and larger-scale commercialization in the form of pulp (Sacramento and Sousa, 2000).
Also, belonging to the family Anacardiaceae and originating from India, the mango was introduced to Brazil by the Portuguese in the 16th century (Pinto, 2011). The chemical composition of mango varies according to the culture conditions, variety, stage of maturity and other factors, and is composed primarily of water, carbohydrates, organic acids, minerals, proteins, vitamins (A, C and B-complex) and pigments, in addition to tannins. Tannins constitute 12 to 18% of mango pits (Rozane et al., 2004).
Wastes from the processing of hog-plum and mango produced by agribusinesses such as fruit pulp and juice, as they are rich in amino acids and tannins, may represent an excellent source of carbon for the production of tannase by SSF. Currently, such wastes are commonly disposed of in the environment, without prior treatment, and become an environmental pollutant. The interest in solid state fermentation for the production of compounds of commercial importance is a consequence of the demand for lower-cost inputs (Chávez-González et al., 2012).
P. rolfssi has already been reported as a good producer of tannase by Bradoo et al. (1996), with 9.24 U mL-l, by means of SmF, however, in this study, by SSF, P. rolfssi URM6216 produced 89.49 U mL-l, surpassing the result previously published.
Batra and Saxena (2005) tested 24 species of Penicillium for their ability to produce tannase in a solid medium containing salts, adding 1% tannic acid, only seven of them did not hydrolyze tannic acid. Among the species tested by Batra and Saxena (2005), P. aurantiogriseum, P. chrysogenum, P. corylophillum, P. rolfssi and P. restrictum presented a hydrolysis zone, corroborating the present study, where the same species were also tested, degrading the acid contained in solid medium. However, the authors tested P. oxalicum, which in their study did not produce a hydrolysis zone, while the same species isolated and tested in this study showed the ability to degrade tannic acid, indicating that the enzyme production capacity is not inherent in the species, but to the isolate (Table 1).
Despite being a traditional method, the a-priori selection for detection of enzyme production using microorganisms inoculated in solid media specific to the production of the desired enzyme in Petri dishes is a flawed method. In the present study, using a qualitative assessment, P. janthinelum, P. lapidosum and P. lanosum did not hydrolyze tannic acid, however, by SSF using mango residue as substrate, they produced 85.93; 18.84 and 30.99 U mL-l tannase, respectively, a fact that proves the failure of the qualitative method.
In 2005, Banerjee et al. (2005) evaluated tannase production by consortium between Rhizopus oryzae (RO IIT RB-13, NRRL 21498) and Aspergillus foetidus (GMRB013 MTCC) by solid state fermentation. The authors observed a maximum yield of 41.3 U mL-l. According to the authors, the consortium between microorganisms enhances the production of the enzyme. However, in this study, in which species of Penicillium were tested separately, a greater yield of tannase was observed, indicating that the genus can be the most promising for the production of the enzyme.
Evaluating the production of tannase by Aspergillus tamarii, through SmF, Costa et al. (2008), using three media containing salts and either tannic acid, gallic acid or methyl gallate as inducers, verified that the culture produced tannase after 48 h of fermentation, in the three inducer media. However, maximum production took place using gallic acid as an inducer (20.6 U mL-l). In literature the Aspergillus genus is recognized as the best tannase producer. The present study confirms the high potential of the genus Penicillium for the production of tannase, which can overcome the production of this enzyme by Aspergillus species. This fact can be observed when comparing the present study (89.48 U mL-l) to the results presented by Costa et al. (2008), who found only 20.6 U mL-l of tannase after 48 h of fermentation by A. tamarii.
Selwal and Selwal (2011) evaluated the production of tannase by an isolate of Penicillium atramentosum, coming from tannery effluent, through submerged fermentation using leaves of “amla” (Phyllanthus emblica), "ber" (Zyzyphus mauritiana), "jamoa" (Eugenia cuspidata), "jamun" (Syzygium cumini) and "keekar" (Acacia nilotica) powder. The authors observed a maximum yield of tannase of 32.8 and 34.7 U mL-l from the leaves of amla (2% w/v) and "keekar" (3% w/v), respectively, incubated at 30°C for 72 h. Comparing with the present study, mango residue proved more promising for the production of tannase, as it had a maximum activity of 89.48 U mL-l. In addition according to Pinto et al. (2005a), solid state fermentation is more advantageous than submerged fermentation, mainly because of the water savings and simplicity of culture medium.
Brazil is a country which is recognized worldwide for its agricultural practice. However the accumulation of residues rich in tannins and their derivatives, such as those of mango, constitutes a serious environmental problem. In this context, P. rolfssi, a microorganism not reported as pathogenic and able to produce tannase by SSF using mango waste, is being indicated as an excellent tool to be used by the food and pharmaceutical industries for the production of tannase. This is the first study to use this residue for the production of tannase.
The authors have not declared any conflict of interests.
REFERENCES
Aguilar CN, Rodríguez R, Gutiérrez-Sánchez G, Augur C, Favela-Torres E, Prado-Barragan LA, Ramírez-Coronel A, Contreras-Esquivel JC (2007). Microbial tannases: advances and perspectives. Appl. Microbiol. Biotechnol. 76:47-59.
Crossref |
|
|
Banerjee R, Mukherjee G, Patra KC (2005). Microbial transformation of tannin-rich substrate to gallic acid through co-culture method. Bioresour. Technol. 96(8):949-953.
Crossref |
|
|
Batra A, Saxena RK (2005). Potential tannase producers from the genera Aspergillus and Penicillium. Process. Biochem. 40:1553-1557.
Crossref |
|
|
|
Battestin V, Matsuda LK, Macedo GA (2004). Fontes e aplicações de taninos e tanases em alimentos. Alim. Nutr. 15(1):63-72. |
|
|
Belur PD, Mugeraya G (2011). Microbial Production of Tannase: State of the Art. Res. J. Microbiol. 6(1):25-40.
Crossref |
|
|
|
Bon EPS, Ferrara MA, Corvo ML (2008). Enzimas em Biotecnologia Produção, aplicações e Mercado. Editora Interciência, Rio de Janeiro. |
|
|
|
Bosco J, Soares KT, Aguiar Filho SP, Barros RV (2000). A cultura da cajazeira. EMEPA, João Pessoa. |
|
|
Bradoo S, Gupta R, Saxena RK (1996). Screening of extracellular tannase-producing fungi: development of a rapid and simple plate assay. J. Gen. Appl. Microbiol. 42:325-329.
Crossref |
|
|
Carvalho JLN, Avanzi JC, Silva MLN, Mello CR, Cerri CEP (2010). Potencial de sequestro de carbono em diferentes biomas do Brasil. Rev. Bras. Cienc. Solo 34(2):277-289.
Crossref |
|
|
Chávez-González M, Rodríguez-Durán LV, Balagurusamy N, Prado-Barragán A, Rodríguez R, Contreras JC, Aguilar CN (2012). Biotechnological Advances and Challenges of Tannase: An Overview. Food Bioproc. Technol. 5:445-459.
Crossref |
|
|
|
Costa AM, Kadowaki MK, Minozzo MC, Souza CGM, Boer, CG, Bracht A,Peralta RM (2012). Production, purification and characterization of tannase from Aspergillus tamari. Afr. J. Biotechnol. 11(2):391-398. |
|
|
Costa AM, Ribeiro WX, Kato E, Monteiro ARG, Peralta RM (2008). Production of tannase by Aspergillus tamarii in Submerged Cultures. Braz. Arch. Biol. Technol. 51:399-404.
Crossref |
|
|
Cruz R, Fonseca JC, Fernandes MJS, Lima DMM, Duda GP, Moreira KA, Souza-Motta CM (2013b). Diversity of Filamentous Fungi of Area from Brazilian Caatinga and High-Level Tannase Production Using Mango (Mangifera indica L.) and Surinam Cherry (Eugenia uniflora L.) Leaves under SSF. Adv. Microbiol. 3:52-60.
Crossref |
|
|
Cruz R, Santos C, Lima JS, Fernandes MJS, Moreira KA, Souza-Motta CM (2013a). Diversity of Penicillium in soil of Caatinga and Atlantic Forest areas of Pernambuco, Brazil: an ecological approach. Nova Hedwigia 97(3-4):543-556.
Crossref |
|
|
Gois IB, Ferreira, RA, Silva-Mann R, Blank MFA, Neto EMS (2014). Diversidade genética entre indivíduos de spondias lutea l. procedentes do baixo são francisco sergipano, por meio de marcadores RAPD. Rev. Árvore 38(2):261-269.
Crossref |
|
|
Govindarajan RK, Revathi S, Rameshkumar, N, Krishnan M, Kayalvizhi, N (2016). Microbial tannase: Current perspectives and biotechnological advances. Biocatal. Agric. Biotechnol. 6:168-175.
Crossref |
|
|
|
Hagerman AE, Butler LG (1981). The specificity of proanthocyanidin-protein interactions. J. Biol. Chem. 256:4494-4497. |
|
|
Lejon DPH, Chaussod R, Ranger J, Ranjard L (2005) Microbial community structure and density under different tree species in an acid forest (Morvan, France). Microbiol. Ecol. 50:614-625.
Crossref |
|
|
Lima JS, Cruz R, Fonseca JC, Medeiros EV, Maciel MHC, Moreira KS, Souza-Motta CM (2014). Production, Characterization of Tannase from Penicillium montanense URM 6286 under SSF Using Agroindustrial Wastes, and Application in the Clarification of Grape Juice (Vitis vinifera L.). Sci. World J. 182025:1-9.
Crossref |
|
|
Madeira Jr JV, Macedo JA, Macedo GA (2012). A new process for simultaneous production of tannase and phytase by Paecilomyces variotii in solid-state fermentation of orange pomace. Bioproc. Biosyst. Eng. 35:477-482.
Crossref |
|
|
Monteiro JMM, Albuquerque UP, Araújo EL, Amorim ELC (2005). Taninos: uma abordagem da química à ecologia. Quim. Nova 28(5):892-896.
Crossref |
|
|
Murugan KS, Saravanababu M, Arunachalam M (2007). Screening of tannin acyl hydrolase (E.C.3.1.1.20) producing tannery effluent fungal isolates using simple agar plate and SmF process. Bioresour. Technol. 98:946-949.
Crossref |
|
|
Pandey A (2003). Solid state fermentation. Biochem. Eng. J. 13(2):81-84.
Crossref |
|
|
|
Pinto ACQ (2016). A produção, o consumo e a qualidade da manga no Brasil. Matéria. Disponível em: http://www.todafruta.com.br/. Accessed in June 04, 2016. |
|
|
|
Pinto GAS, Brito ES, Andrade AMR, Fraga SLP, Teixeira RB (2005a). Fermentação em Estado Sólido: Uma Alternativa para o Aproveitamento e Valorização de Resíduos Agroindustriais Tropicais. EMBRAPA Comunicado técnico online 1:5. |
|
|
Pinto GAS, Couri S, Leite SGF, Brito ES (2005b).). Tanase: conceitos, produção e aplicação. Bol. Centro Pesq. Proces. Alim. 23(2):435-462.
Crossref |
|
|
|
Pitt JI (1991). A laboratory Guide to Common Penicillium Species. Commonwealth Scientific and Industrial Research Organization – Division of Food Processing, North Wales. |
|
|
|
Reis MAR (2007). Unidades de Conservação no Brasil: da república à gestão de classe mundial. Belo Horizonte: SEGRAC. |
|
|
Renovato J, Gutiérrez-Sánchez G, Rodríguez-Durán LV, Bergman C, Rodríguez R, Aguilar CN (2011) Differential Properties of Aspergillus niger Tannase Produced Under Solid-State and Submerged Fermentations. Appl. Biochem. Biotechnol. 165:382-395.
Crossref |
|
|
|
Rozane DE, Darezzo RJ, Aguiar RL, Aguilera GHA, Zambolim L (2004). Manga, produção integrada, industrialização e comercialização. Suprema Gráfica e Editora. |
|
|
Sabu A, Pandey A, Jaafar Daud M, Szakacs G (2005). Tamarind seed powder and palm kernel cake: two novel agroresidues for the production of tannase under solid state fermentation by Aspergillus niger TCC 16620. Bioresour. Technol. 96:1223-1228.
Crossref |
|
|
|
Sacramento CK, Souza FX (2000). Cajá (Spondias mombin L.). Funep, Jaboticabal. |
|
|
|
Samson RA, Frisvad JC (2004). Penicillium Subgenus Penicillium: new Taxonomic Schemes, Mycotoxins and Other Extrolites. Stud. Mycol. 49:1-260. |
|
|
Selwal MK, Selwal KK (2011). High-level tannase production by Penicillium atramentosum KM using agro residues under submerged fermentation. Ann. Microbiol. 62(1):139-148.
Crossref |
|
|
Selwal MK, Yadav A, Selwal KK, Aggarwal NK, Gu
|
Aguilar CN, Rodríguez R, Gutiérrez-Sánchez G, Augur C, Favela-Torres E, Prado-Barragan LA, Ramírez-Coronel A, Contreras-Esquivel JC (2007). Microbial tannases: advances and perspectives. Appl. Microbiol. Biotechnol. 76:47-59.
Crossref
|
|
|
|
Banerjee R, Mukherjee G, Patra KC (2005). Microbial transformation of tannin-rich substrate to gallic acid through co-culture method. Bioresour. Technol. 96(8):949-953.
Crossref
|
|
|
|
|
Batra A, Saxena RK (2005). Potential tannase producers from the genera Aspergillus and Penicillium. Process. Biochem. 40:1553-1557.
Crossref
|
|
|
|
|
Battestin V, Matsuda LK, Macedo GA (2004). Fontes e aplicações de taninos e tanases em alimentos. Alim. Nutr. 15(1):63-72.
|
|
|
|
|
Belur PD, Mugeraya G (2011). Microbial Production of Tannase: State of the Art. Res. J. Microbiol. 6(1):25-40.
Crossref
|
|
|
|
|
Bon EPS, Ferrara MA, Corvo ML (2008). Enzimas em Biotecnologia Produção, aplicações e Mercado. Editora Interciência, Rio de Janeiro.
|
|
|
|
|
Bosco J, Soares KT, Aguiar Filho SP, Barros RV (2000). A cultura da cajazeira. EMEPA, João Pessoa.
|
|
|
|
|
Bradoo S, Gupta R, Saxena RK (1996). Screening of extracellular tannase-producing fungi: development of a rapid and simple plate assay. J. Gen. Appl. Microbiol. 42:325-329.
Crossref
|
|
|
|
|
Carvalho JLN, Avanzi JC, Silva MLN, Mello CR, Cerri CEP (2010). Potencial de sequestro de carbono em diferentes biomas do Brasil. Rev. Bras. Cienc. Solo 34(2):277-289.
Crossref
|
|
|
|
|
Chávez-González M, Rodríguez-Durán LV, Balagurusamy N, Prado-Barragán A, Rodríguez R, Contreras JC, Aguilar CN (2012). Biotechnological Advances and Challenges of Tannase: An Overview. Food Bioproc. Technol. 5:445-459.
Crossref
|
|
|
|
|
Costa AM, Kadowaki MK, Minozzo MC, Souza CGM, Boer, CG, Bracht A,Peralta RM (2012). Production, purification and characterization of tannase from Aspergillus tamari. Afr. J. Biotechnol. 11(2):391-398.
|
|
|
|
|
Costa AM, Ribeiro WX, Kato E, Monteiro ARG, Peralta RM (2008). Production of tannase by Aspergillus tamarii in Submerged Cultures. Braz. Arch. Biol. Technol. 51:399-404.
Crossref
|
|
|
|
|
Cruz R, Fonseca JC, Fernandes MJS, Lima DMM, Duda GP, Moreira KA, Souza-Motta CM (2013b). Diversity of Filamentous Fungi of Area from Brazilian Caatinga and High-Level Tannase Production Using Mango (Mangifera indica L.) and Surinam Cherry (Eugenia uniflora L.) Leaves under SSF. Adv. Microbiol. 3:52-60.
Crossref
|
|
|
|
|
Cruz R, Santos C, Lima JS, Fernandes MJS, Moreira KA, Souza-Motta CM (2013a). Diversity of Penicillium in soil of Caatinga and Atlantic Forest areas of Pernambuco, Brazil: an ecological approach. Nova Hedwigia 97(3-4):543-556.
Crossref
|
|
|
|
|
Gois IB, Ferreira, RA, Silva-Mann R, Blank MFA, Neto EMS (2014). Diversidade genética entre indivíduos de spondias lutea l. procedentes do baixo são francisco sergipano, por meio de marcadores RAPD. Rev. Árvore 38(2):261-269.
Crossref
|
|
|
|
|
Govindarajan RK, Revathi S, Rameshkumar, N, Krishnan M, Kayalvizhi, N (2016). Microbial tannase: Current perspectives and biotechnological advances. Biocatal. Agric. Biotechnol. 6:168-175.
Crossref
|
|
|
|
|
Hagerman AE, Butler LG (1981). The specificity of proanthocyanidin-protein interactions. J. Biol. Chem. 256:4494-4497.
|
|
|
|
|
Lejon DPH, Chaussod R, Ranger J, Ranjard L (2005) Microbial community structure and density under different tree species in an acid forest (Morvan, France). Microbiol. Ecol. 50:614-625.
Crossref
|
|
|
|
|
Lima JS, Cruz R, Fonseca JC, Medeiros EV, Maciel MHC, Moreira KS, Souza-Motta CM (2014). Production, Characterization of Tannase from Penicillium montanense URM 6286 under SSF Using Agroindustrial Wastes, and Application in the Clarification of Grape Juice (Vitis vinifera L.). Sci. World J. 182025:1-9.
Crossref
|
|
|
|
|
Madeira Jr JV, Macedo JA, Macedo GA (2012). A new process for simultaneous production of tannase and phytase by Paecilomyces variotii in solid-state fermentation of orange pomace. Bioproc. Biosyst. Eng. 35:477-482.
Crossref
|
|
|
|
|
Monteiro JMM, Albuquerque UP, Araújo EL, Amorim ELC (2005). Taninos: uma abordagem da química à ecologia. Quim. Nova 28(5):892-896.
Crossref
|
|
|
|
|
Murugan KS, Saravanababu M, Arunachalam M (2007). Screening of tannin acyl hydrolase (E.C.3.1.1.20) producing tannery effluent fungal isolates using simple agar plate and SmF process. Bioresour. Technol. 98:946-949.
Crossref
|
|
|
|
|
Pandey A (2003). Solid state fermentation. Biochem. Eng. J. 13(2):81-84.
Crossref
|
|
|
|
|
Pinto ACQ (2016). A produção, o consumo e a qualidade da manga no Brasil. Matéria. Disponível em: http://www.todafruta.com.br/. Accessed in June 04, 2016.
|
|
|
|
|
Pinto GAS, Brito ES, Andrade AMR, Fraga SLP, Teixeira RB (2005a). Fermentação em Estado Sólido: Uma Alternativa para o Aproveitamento e Valorização de Resíduos Agroindustriais Tropicais. EMBRAPA Comunicado técnico online 1:5.
|
|
|
|
|
Pinto GAS, Couri S, Leite SGF, Brito ES (2005b).). Tanase: conceitos, produção e aplicação. Bol. Centro Pesq. Proces. Alim. 23(2):435-462.
Crossref
|
|
|
|
|
Pitt JI (1991). A laboratory Guide to Common Penicillium Species. Commonwealth Scientific and Industrial Research Organization – Division of Food Processing, North Wales.
|
|
|
|
|
Reis MAR (2007). Unidades de Conservação no Brasil: da república à gestão de classe mundial. Belo Horizonte: SEGRAC.
|
|
|
|
|
Renovato J, Gutiérrez-Sánchez G, Rodríguez-Durán LV, Bergman C, Rodríguez R, Aguilar CN (2011) Differential Properties of Aspergillus niger Tannase Produced Under Solid-State and Submerged Fermentations. Appl. Biochem. Biotechnol. 165:382-395.
Crossref
|
|
|
|
|
Rozane DE, Darezzo RJ, Aguiar RL, Aguilera GHA, Zambolim L (2004). Manga, produção integrada, industrialização e comercialização. Suprema Gráfica e Editora.
|
|
|
|
|
Sabu A, Pandey A, Jaafar Daud M, Szakacs G (2005). Tamarind seed powder and palm kernel cake: two novel agroresidues for the production of tannase under solid state fermentation by Aspergillus niger TCC 16620. Bioresour. Technol. 96:1223-1228.
Crossref
|
|
|
|
|
Sacramento CK, Souza FX (2000). Cajá (Spondias mombin L.). Funep, Jaboticabal.
|
|
|
|
|
Samson RA, Frisvad JC (2004). Penicillium Subgenus Penicillium: new Taxonomic Schemes, Mycotoxins and Other Extrolites. Stud. Mycol. 49:1-260.
|
|
|
|
|
Selwal MK, Selwal KK (2011). High-level tannase production by Penicillium atramentosum KM using agro residues under submerged fermentation. Ann. Microbiol. 62(1):139-148.
Crossref
|
|
|
|
|
Selwal MK, Yadav A, Selwal KK, Aggarwal NK, Gupta R, Gautam SK (2011). Tannase production by Penicillium atramentosum km under SSF and its applications in wine clarification and tea cream solubilization. Braz. J. Microbiol. 42:374-387.
Crossref
|
|
|
|
|
Sharma S, Bhat TK, Dawra RK (2000). A Spectrophotometric Method for Assay of Tannase Using Rhodanine. Anal. Biochem. 279:85-89.
Crossref
|
|
|
|
|
Singh JS, Pandey VC, Singh DP (2011). Efficient soil microorganisms: A new dimension for sustainable agriculture and environmental development. Agric. Ecosyst. Environ. 140:339-353.
Crossref
|
|
pta R, Gautam SK (2011). Tannase production by Penicillium atramentosum km under SSF and its applications in wine clarification and tea cream solubilization. Braz. J. Microbiol. 42:374-387.
Crossref |
|
|
Sharma S, Bhat TK, Dawra RK (2000). A Spectrophotometric Method for Assay of Tannase Using Rhodanine. Anal. Biochem. 279:85-89.
Crossref |
|
|
Singh JS, Pandey VC, Singh DP (2011). Efficient soil microorganisms: A new dimension for sustainable agriculture and environmental development. Agric. Ecosyst. Environ. 140:339-353.
Crossref |