ABSTRACT
Proteases are important commercial enzymes, and among their numerous sources are the Basidiomycetes. The use of proteases in many industrial areas promotes the search for enzymes with new properties. The aim of this study is to produce and characterize peptidases of a biocomposite from mycelial biomass grown in Amazonic tubers. Lentinus citrinus DPUA 1535 and Pleurotus ostreatoroseus DPUA 1720 were cultivated on Dioscorea trifida, Manihot esculenta and Dioscorea alata supplemented with rice bran or manioc flour residue in different proportions. The highest proteolytic activity was determined in the crude extract from P. ostreatoroseus grown in D. alata (DA) without supplementation (142.22 U/mL). The enzymes showed optimum activities at 40°C and pH 7.0; and stability at 50°C and pH 8.0. The proteases were classified as cysteine proteases based on the effect of inhibitors used.
Key words: Pleurotus, Lentinus, Dioscorea, protease.
Proteases are enzymes with important biotechnological use. They have applications in chemical and biochemical reaction on food, beverages, pharmaceutical products and cosmetics. Proteases represent one of the biggest groups of world industrial enzymes with perspective increasing around 7% until 2020 (Singh et al., 2016; Geng et al., 2016; Chandrasekaran et al., 2015). Peptidases can be obtained from microorganism (bacteria and fungi), plants or animals. However, the microbial sources of these enzymes have preference to be used as protease producers due to their physiological and biochemical properties, suitability to genetic manipulation and short time of fermentation process (Sharma et al., 2017; Souza et al., 2016).
The edible mushrooms, ​Lentinus crinitus​, ​Lentinus citrinus​, ​Pleurotus ostreatoroseus​, ​ Pleurotus florida and Pleurotus albidus have been reported as enzymes sources, including proteases (Fonseca et al., 2014; Kirsch et al., 2011; Martim et al., 2017; Souza et al., 2016)​. Edible mushrooms are consumed by many civilizations for centuries due to their nutritional and dietetic properties. They present high quantities of protein, fiber and low levels of fats and produce enzymes, vitamins, antimicrobial compounds, antioxidants and immune stimulants (Reid et al., 2017). Edible mushrooms promote important benefit to health due to their nutritional composition. Their protein content is similar to the ones from animal and plant sources and higher than most of other food. Edible mushrooms contain all essential amino acids that are required in human diet (Nwoko et al., 2017).
The cultivation of mushrooms to produce biocompounds from the combination of mycelium and substrates of different compositions allows one to obtain many compounds of biological activity (Haneef et al., 2017). Pulp of tubers of Dioscorea trifida (cará-roxo), Dioscorea alata (inhame roxo) and Manihot esculenta (macaxeira) can serve as substrates with nutritional properties that can be used to cultivate edible mushrooms and also for the production of biocatalysts as peptidases. Considering the availability of edible tubers in Amazon, this research aims to produce and characterize peptidases of a biocomposite from mycelia biomass grown in Amazonic tubers.
Edible mushrooms
L. citrinu​s DPUA 1535 and P. ostreatoroseus DPUA 1720 were theselected edible mushrooms species for this study (Micoteca DPUA, Federal University of Amazonas- UFAM). The mushrooms were cultivated on potato dextrose agar (PDA) with 0.5% (w/v) yeast extract to obtain the matrix culture.
Substrates
The tubers D. trifida (cará-roxo), M. esculenta ​(macaxeira) and D. alata ​(inhame-roxo) were obtained from a local market of Manaus (Amazonas/Brazil). They were washed and sanitized in sodium hypochlorite (50 ppm) for 10 min. The peel was removed and the tubers were cut in cubes of 1 cm, distributed in polyethylene bags and sterilized at 121°C for 10 min (Brasil, 2007).
Inoculum selection
The inoculum was chosen from selection of culture media. The mushrooms were cultivated on potato agar dextrose with 0.5% (w/v) yeast extract (PDA) and oat bran agar with 0.1% yeast extract (OMYA) distributed in Petri dishes. The cultures were maintained at 25°C for 8 days in the absence of light. The selected culture medium was the one that promoted significant radial growth. The radial growth was evaluated by measuring the colony diameter every 24 h until there was complete colonization of medium surface in the dish.
The mycelial vigor was classified by a subjective method of grades: grade 1 weakly dense, grade 2 moderately dense and grade 3 strongly dense (Fonseca et al., 2014). The medium that promoted significant radial growth was used for mushroom cultivation. From this culture, 10 mycelial discs (Ø = 10 mm) were inoculated in 50 mL of glucose, peptone and yeast extract (GYP). The fermentation was carried out at 25°C and 150 rpm. After five days, the biomass was separated from crude extract by filtration using an aluminum sieve (Ø = 75 mm).
Solid state fermentation
The recovered biomass of submerged fermentation was inoculated in the substrates supplemented with rice bran or manioc flour residue (crueira) in different proportions (Table 1). The patterns were the substrates without supplementation. The fermentation was carried out at 25°C, in the absence of light, 60% humidity, until there was complete colonization of the mycelium in the substrates. All the experiments were made in triplicate
After myceliation was completed in the tubers, they were dehydrated at 40°C in forced air oven for 24 h. Then, they were crushed and the granules were standardized with sieve of 10 mesh diameter.
Enzymes extraction and determination of proteolytic activity
The enzymes were extracted in distilled water using the proportion 1:5 (myceliated tuber : water). The mixture was maintained at 25°C, and 150 rpm for 1 h. The crude extract was recovered by vacuum filtration using Whatman no. 1 filter paper. Proteolytic activity was determined according to the methodology described by Leighton et al. (1973). A mixture containing 0.15 mL of crude extract and 0.25 mL substrate [1% (w/w) azocasein in 0.2 M Tris-HCl buffer, pH = 7.2] was incubated for 60 min in the absence of light. The reaction was interrupted by addition of 10% (w/w) trichloroacetic acid and centrifuged (8000 rpm) for 15 min at 4°C. The supernatant (0.8 mL) was added to 1.4 mL of 1 M NaOH. One unit of proteolytic activity was defined as the amount of enzyme that promotes a 0.01 increase of absorbance in one hour at 440 nm.
Effect of pH and temperature on enzyme activity and stability
To assay optimum pH, proteolytic activity was determined at 25°C, with azocasein in different pH ranges using the following 0.1 M buffer solutions: citrate (5.0 and 6.0), phosphate (7.0 and 8.0) and carbonate-bicarbonate (9.0 and 10.0). Optimum temperature was determined by incubating the enzyme extract with azocasein at temperatures ranging from 25 to 80°C and assaying the activity at the pH determined as optimum.
For the pH stability, the crude extract was dispersed (1:1), for one hour, in the following 0.1 M buffer solutions: citrate (5.0 and 6.0), phosphate (7.0 and 8.0) and carbonate-bicarbonate (9.0 and 10.0); it was incubated in azocasein and maintained at optimum temperature for 1 h. For thermal stability study, the enzyme extracts were incubated in azocasein at different temperatures ranging from 25 to 80°C for 1 h. All samples were prepared in triplicate.
Effect of protease inhibitors and metal ions on enzyme activity
The e​ff​ect of inhibitors and metal ions on enzyme activity was investigated by using 10 mM of calcium chloride (CaCl​2)​, potassium chloride (KCl), sodium chloride (NaCl), copper sulphate (CuSO4)​, ferrous sulphate (FeSO​4​), zinc sulphate (ZnSO4) and protease inhibitor compounds such as phenyl-methylsulfonyl fluoride (PMSF), ethylene-diaminetetraacetic acid (EDTA), iodoacetic acid and pepstatin A (10 mM). The crude extracts were incubated with the solutions of ions and inhibitors at 50°C for 1 h. After this time, they were incubated in 1% (w/v) azocasein at 40°C for 60 min, in the absence of light. Residual enzyme activities were determined and compared with the control which was incubated without the inhibitors (0% inhibition) and metal ions and corresponds to 100% of enzyme activity. All samples were prepared in triplicate (Alecrim et al., 2015).
Inoculum selection
Table 2 shows the results of radial growth of ​L. citrinus ​and ​P. ostreatoroseus ​in solid medium, for six days. The significant value of growth and higher mycelial density in both cultures was observed in oat bran agar and yeast extract (OMYA+YE) medium (67.0 and 65.5 mm, respectively). L. citrinus ​presented moderately dense white mycelium in OMYA+YE and in potato dextrose agar and yeast extract (PDA+YE), while ​P. ostreatoroseus ​presented moderately dense white mycelium in PDA+YE and strongly dense pinkish mycelium in OMYA+YE. According to these results, OMYA+YE medium was considered appropriate for the growth of both cultures in the analyzed conditions. The results are similar to other studies that presented mushroom growth and morphology in pure culture using different culture media. This proves that this condition influences fungi growth (Sastre-Ahuatzi et al., 2007; Wiriya et al., 2014; Masoumi et al., 2015).
The effect of different media cultures on the mycelial growth of basidiomycetes was reported in the study of Okwulehie and Okwujiako (2008). They observed that OMYA stimulated the growth of ​P. ostreatus ​var. ​florida ​Eger. Lentinula edodes presented a dense mycelium when cultivated on OMYA in the study of Escobar et al. (2007). The oat is considered a food with high nutritional value containing carbohydrates, amino acids, minerals and vitamins (Rasane et al., 2015). The in vitro ​cultivation aims to clarify the optimum conditions of fungi species growth related to availability of nutrients in the medium culture, temperature and time of incubation. This knowledge is an important prerequisite to possible cultivation in large scale (Andrade et al., 2010).
Proteolytic activity
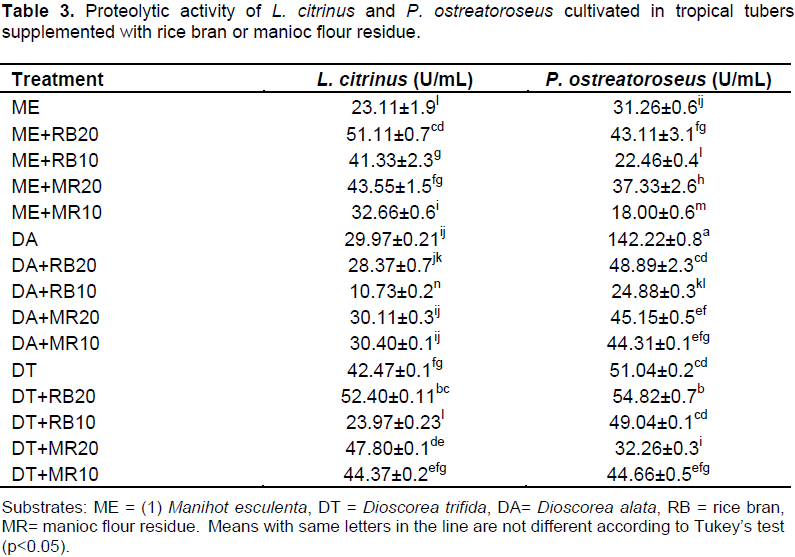
The proteolytic activity of the extracts obtained from L.​ citrinus ​and P.​ ostreatoroseus solid fermentation in different mixtures of tubers and supplements is shown in Table 3. In all studied conditions of solid fermentation, proteases were produced, but the protease activity was different according to the type of supplementation and fungi species. The highest proteolytic activity was determined from P.​ ostreatoroseus ​grown in D. alata ​(DA) without supplementation (142.22 U/mL), while the lowest proteolytic activity (24.88 U/mL) was determined in M. esculenta ​supplemented with 10% of manioc residue (ME+MR 10). L.​ citrinus ​presented high proteolytic activity in DF+RB 20 (52.40 U/mL) and low in DA+RB 10 (10.73 U/mL). The results of this study with P.​ ostreatoroseus were higher than the ones reported by Fonseca et al. (2014), who observed significant proteolytic activity (7.89 U/mL) from P.​ ostreatoroseus ​using cupuaçu exocarp supplemented with 20% rice bran as substrate. Similar results were reported by Machado et al. (2016) and Souza et al. (2016) that cultivated L.​ citrinus on cupuaçu exocarp supplemented with litter and pineapple bark without supplementation, respectively. The results of this investigation revealed that the use of the tubers, especially D. alata, as substrates promoted the production of proteases by P. ostreatoroseus and L. citrinus.
Effect of pH and temperature on enzyme activity and stability
The proteolytic enzymes presented activity in all pH ranges analyzed. However, the optimum activity was observed at pH 7.0, maintaining around 88% of activity at pH 6.0. These results are in agreement with the ones reported by Fonseca et al. (2014) who cultivated P. ostreatoroseus on amazonic substrates (sawdust, açai seeds, cupuaçu exocarp, pineapple peel and pineapple pulp) and observed optimum activity of the enzymes at
pH 6.0 to 7.0.
The production of extracellular proteases from P. ostreatorouseus cultivated on DA is probably associated with the mushroom that needs to be hydrolyzed in different types of substrates as nutritional source of protein. Nirma et al. (2011) reported that fungi can produce acid, neutral and alkaline proteases. One single species is capable to produce more than one kind of these enzymes with optimum activity in a wide range of
pH (4.0 to 11.0).
P. ostreatoroseus also showed activity in all
temperatures tested. But, the optimum activity was observed at 40°C. From this temperature, there was an increased activity (Figure 1B). At high temperatures, the enzymes suffer protein denaturation because the intramolecular bonds are affected (Ahmed et al., 2011). In the studies of Fonseca et al. (2014), Guan et al. (2011) and Machado et al. (2016), the optimum temperature activity of the mushrooms P. ostreatoroseus, Pholiota nameko and L. citrinus was also determined at 40 and 50°C, respectively.
The stability of pH and temperature is an important parameter in the enzymes application due to their determination of economic availability in the industrial processes (Moretti et al., 2012). The proteases of P. ostreatoroseus maintained stability at pH 7.0 and 8.0 with relative activity of 95 and 100%, respectively, for 60 min. The reduction of pH stability was observed at pH 9.0 (Figure 2A). At 40 and 50°C, the stability was maintained in 94 and 100% during 60 min. The inactivation of the enzymes was determined at 70°C (Figure 2B). According to Cheng et al. (2012), the thermostability of mushrooms proteolytic enzymes can be variable. Proteases from L. citrinus cultivated on cupuaçu exocarp and litter were active in all the temperatures tested. However, at 30°C they exhibited high activity for 60 min (Machado et al., 2016).
Effect of protease inhibitors and metal ions on enzyme activity
The enzymes of P. ostreatoroseus were inhibited at 95,94 and 87% by iodine acetic acid, PMSF and EDTA, respectively. These results suggest that the proteases be classified as cysteine, metalo and serine proteases. Some studies report the production of different types of proteases by mushrooms. Lebedeva and Proskuryakov (2009) and Zhang et al. (2010) observed inhibition in the proteases activity of P. ostreatus (Fr.) Kumm and Hypsizigus marmoreus, respectively using PMSF. This suggests the presence of serine proteases.
Based on the effect of metallic ions on the activity of P. ostreatoroseus enzymes, the ions Cu2+ and Zn2+ caused a reduction of 95% (Table 4). However, at similar conditions, the ions K+, Mg2+ and Ca2+ did not have high influence on the proteases activity. Martim et al. (2017) showed that Zn2+ increased the activity of P. albidus enzymes at 78%. Ahmed and Helmy (2012) also observed the influence of Zn2+ at 67.7% with Bacillus licheniformis 5A5 enzymes. Couto and Sanromán (2006) showed that the interaction of metallic ions with fungi enzymes white rot is particularly important to the comprehension of biotechnology processes regulation of fungi degradation. The metallic ions can bond to amino acid residues and modify the protein structure that can have positive or negative proteolytic activity (Merheb-Dini et al., 2010).
The significant values of proteolytic activity were determined in the bioproduct from P. ostreatoroseus myceliation in D. alata tuber. In the experimental conditions, the data suggested the predominant presence of cysteine ande serine proteases. The protease expressed optimum activity at 40 °C and pH 7.0 with highest stability at 50 °C and pH 8.0.
The authors have not declared any conflict of interests.
REFERENCES
|
Ahmed I, Zia MA, Iftikhar T, Iqbal HM (2011). Characterization and detergent compatibility of purified protease produced from Aspergillus niger by utilizing agro wastes. BioResources 6(4):4505-4522.
|
|
|
|
Ahmed SA, Helmy WA (2012). Comparative evaluation of Bacillus licheniformis 5A5 and Aloe variegata milk-clotting enzymes. Braz. J. Chem. Eng. 29(1):69-76.
Crossref
|
|
|
|
|
Alecrim MM, Palheta RA, Teixeira MFS, Oliveira IMDA (2015). Milkâ€clotting enzymes produced by Aspergillus flavo furcatis strains on Amazonic fruit waste. Int. J. Food Sci. Technol. 50(1):151-157.
Crossref
|
|
|
|
|
Andrade MCN, Chavari JL, Minhoni MTA, Zied DC (2010). Crescimento micelial in vitro de cinco linhagens de Agaricus bisporus submetidas a diferentes condições de temperatura. Acta Sci. Agron. 32(1):69-72.
Crossref
|
|
|
|
|
Brasil (2007). Agência Nacional de Vigilância Sanitária. Higienização das mãos em serviços de saúde/ Agência Nacional de Vigilância
|
|
|
|
|
Chandrasekaran S, Kumaresan SSP, Manavalan M (2015). Production and Optimization of Protease by Filamentous Fungus Isolated from Paddy Soil in Thiruvarur District Tamilnadu. J. Appl. Biol. Biotechnol. 3(6):66-69.
|
|
|
|
|
Cheng S, Xu J, Liu C, Zhu Y, Nelson DR, Zhou S, Luo H (2012). Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat. Commun. 3(913):1-12.
|
|
|
|
|
Couto SR, Sanromán MA (2006). Application of solid-state fermentation to food industry-A review. J. Food Eng. 76(3):291-302.
Crossref
|
|
|
|
|
Escobar VV, Pérez AM, Arredondo C (2007). Evaluación de la producción del hongo Lentinula edodes Pegler en bloques sintéticos a base de residuos agroindustriales. Ingeniería y Ciencia 3(6):23-39.
|
|
|
|
|
Fonseca TRB, Barroncas JF, Teixeira MFS (2014). Produção em matriz sólida e caracterização parcial das proteases de cogumelo comestível da Floresta Amazônica. Rev. Bras. Tecnol. Agroind. 8(1):1227-1236.
Crossref
|
|
|
|
|
Geng C, Nie X, Tang Z, Zhang Y, Lin J, Sun M, Peng D (2016). A Novel Serine Protease, Sep1, From Bacillus Firmus DS-1 Has Nematicidal Activity and Degrades Multiple Intestinal-Associated Nematode Proteins. Sci. Rep. 6:25012.
Crossref
|
|
|
|
|
Guan GP, Zhang GQ, Wu YY, Wang HX, Ng TB (2011). Purification and characterization of a novel serine protease from the mushroom Pholiota nameko. J. Bio. Bioeng. 111(6):641-645.
Crossref
|
|
|
|
|
Haneef M, Ceseracciu L, Canale C, Bayer IS, Heredia-Guerrero JA, Athanassiou A (2017). Advanced Materials From Fungal Mycelium: Fabrication and Tuning of Physical Properties. Sci. Rep. 7(41292):1-11.
Crossref
|
|
|
|
|
Kirsch LS, Pinto ACP, Porto TS, Porto ALF, Teixeira MFS (2011). The influence of different submerged cultivation conditions on mycelial biomass and protease production by Lentinus citrinus Walleyn et Rammeloo DPUA 1535 (Agaricomycetideae). Int. J. Med. Mushrooms 13(2):185-192.
Crossref
|
|
|
|
|
Lebedeva GV, Proskuryakov MT (2009). Purification and characterization of milk-clotting enzymes from oyster mushroom (Pleurotus ostreatus (Fr.) Kumm). Appl. Biochem. Microbiol. 45(6):623.
Crossref
|
|
|
|
|
Leighton TJ, Doi RH, Warren RAJ, Kelln RA (1973). The relationship of serine protease activity to RNA poly-merase modification and sporulation in Bacillus subtilis. J. Mol. Biol. 76:103-122.
Crossref
|
|
|
|
|
Machado ARG, Teixeira MFS, Kirsch LS, Campelo MDCL, Oliveira IMA (2016). Nutritional value and proteases of Lentinus citrinus produced by solid state fermentation of lignocellulosic waste from tropical region. Saudi J. Biol Sci. 23(5):621-627.
Crossref
|
|
|
|
|
Martim SR, Silva LSC, Souza LB, Carmo EJ, Alecrim MM, Vasconcellos MC, Teixeira MFS (2017). Pleurotus albidus: A new source of milk-clotting proteases. Afr. J. Microbiol Res. 11(17):660-667.
Crossref
|
|
|
|
|
Masoumi F, Pourianfar HR, Masoumi A, MostafaviMendi E (2015). A study of mycelium characterization of several wild genotypes of the button mushroom from Iran. Int. J. Adv. Res. 3:236-246.
|
|
|
|
|
Merheb-Dini C, Gomes E, Boscolo M, da Silva R (2010). Production and characterization of a milk-clotting protease in the crude enzymatic extract from the newly isolated Thermomucor indicae-seudaticae N31:(Milk-clotting protease from the newly isolated Thermomucor indicae-seudaticae N31). Food Chem. 120(1):87-93.
Crossref
|
|
|
|
|
Moretti M, Bocchini-Martins DA, Silva RD, Rodrigues A, Sette LD, Gomes E(2012). Selection of thermophilic and thermotolerant fungi for the production of cellulases and xylanases under solid-state fermentation. Braz. J. Microbiol. 43(3):1062-1071.
Crossref
|
|
|
|
|
Nirma NP, Shankar S, Laxman RS (2011). Fungal proteases: An overview. Int. J. Biotechnol. Biosci. 1(1):1-40.
|
|
|
|
|
Nwoko MC, Onyeizu UR, Okwulehie IC, Ukoima HN (2017). Nutritional and Bioactive Compounds: Evaluation of Pleurotus pulmonarius (Fries) Quel. Fruit Bodies Grown on Different Wood Logs in Abia State, Nigeria. J. Bioremediat. Biodegrad. 8:393.
|
|
|
|
|
Okwulehie IK, Okwujiako IA (2008). The Effects of Some Physical and Nutritional Factors on the Vegetative Growth of Pleurotus ostreatus var. Florida Eger. Under Tropical Conditions. Dyn. Biochem. Process Biotechnol. Mol. Biol. 2(1):41-44.
|
|
|
|
|
Rasane P, Jha A, Sabikhi L, Kumar A, Unnikrishnan VS (2015). Nutritional advantages of oats and opportunities for its processing as value added foods-a review. J. Food Sci. Technol. 52(2):662-675.
Crossref
|
|
|
|
|
Reid T, Munyanyi M, Mduluza T (2017). Effect of cooking and preservation on nutritional and phytochemical composition of the mushroom Amanita zambiana. Food Sci. Nutr. 5(3):538-544.
Crossref
|
|
|
|
|
Sastre-Ahuatzi M, Téllez-Téllez M, Díaz-Godínez G, Montiel-González AM, Díaz R, Sánchez C (2007). Mycelial growth of strains of Pleurotus ostreatus developed on agar and its correlation with the productivity in pilot production farm. Braz. J. Microbiol. 38(3):568-572.
Crossref
|
|
|
|
|
Sharma KM, Kumar R, Panwar S, Kumar A (2017). Microbial alkaline proteases: Optimization of production parameters and their properties. J. Genet. Eng. Biotechnol. 15:115-126.
Crossref
|
|
|
|
|
Singh R , Kumar M, Mitta A, Mehta PK (2016). Microbial enzymes: Industrial progress in 21st century. 3 Biotechnol. 6(2):174.
|
|
|
|
|
Souza RAT, Fonseca TRB, Kirsch LS, Silva LSC, Alecrim MM, Filho, RFC, Teixeira MFS (2016). Nutritional composition of bioproducts generated from semi-solid fermentation of pineapple peel by edible mushrooms. Afr. J. Biotechnol. 15(12):451-457.
Crossref
|
|
|
|
|
Wiriya J, Kavinlertvatana P, Lumyong S (2014). Effects of different culture media, carbon and nitrogen sources and solid substrates on growth of Termitomyces mushrooms. Chiang Mai J. Sci. 41(3):542-556.
|
|
|
|
|
Zhang X, Liu Q, Zhang G, Wang H, Ng T (2010). Purification and molecular cloning of a serine protease from the mushroom Hypsizigus marmoreus. Proc. Biochem. 45(5):724-730.
Crossref
|
|