Traditional fermented beverages occupy an important place in the diet of populations in Burkina Faso. Zoom-koom in particular remains one of the most consumed cereal-based drinks in cities like Ouagadougou. The drink zoom-koom is consumed primarily in the liquid form and to short scale in the reconstituted form called instant flour zoom-koom. Being able to diversify zoom-koom taste and aroma and to improve the nutritional and hygienic quality would definitely add value to this product. For sensorial analysis, 04 formulations of liquid zoom-koom and instant flour zoom-koom were realized and evalued by a panel of tasters. The best formulation of each type of zoom-koom was produced using Lactobacillus fermentum strains SF9.5 and SF6.2 as starter culture. Then, the microbiological (aerobic mesophilic bacteria, lactic acid bacteria, coliforms, yeasts and molds), physico-chemical (pH, acidity, proteins, sugar, lipids, iron, magnesium, calcium) and sensory parameters of each product were determined. The results showed a marked improvement in protein content (25.83-28.41%) for instant flour zoom-koom, in reducing sugars for liquid zoom-koom (32.1-36.9%) and for instant flour zoom-koom (29.88-47.43%), in calcium (39-43 mg for liquid zoom-koom), in magnesium (59-65 mg for liquid zoom-koom), in iron (2.37-2.50 mg and 3.0-3.08 mg respectively for liquid zoom-koom and instant flour zoom-koom). A reduction in total coliforms from 1.3×104 to 4.0×101 cfu/ml for the liquid zoom-koom and from 4.3×104 to 6.8×103 cfu/ml for the instant flour zoom-koom was observed. In addition, a reduction in mold growth ranging from 1.3×104 to 4.0×101 cfu/ml for the liquid zoom-koom and from 1.3×104 to 7.0×103 cfu/ml for instant flour zoom-koom was observed. The zoom-koom samples using L. fermentum starter cultures were well appreciated by at least 80% of the tasters.
Traditional foods including drinks play a major role in African societies. In West Africa especially in Burkina Faso, traditional cereal-based (sorghum, maize or millet) beverages participate daily to the dietary habits of populations (Barro et al., 2003; Tankoano et al, 2017). Fermentation has long been one of the methods of processing and preserving traditional foods (Yao et al., 2009). Lactic acid bacteria, yeasts and molds were identified as the main microorganisms observed during food fermentation (Yao et al, 2009). Lactic fermentation is known as a biochemical process which can enhance aroma and texture (Tapsoba et al., 2017c). Previous studies on controlled lactic fermentation of traditional zoom-koom-like beverages such as kunun-zaki in Nigeria (Gaffa et al., 2002; Amusa et al., 2009; Agarry et al., 2010) showed at the end of the technological process, a marked improvement in the nutritional, hygienic and organoleptic qualities of kunun-zaki. The utilization of starter cultures in the processing can be a solution for the improvement of nutritionnal, organoleptic and sanitary quality of indigenous foods and beverage such as zoom-koom.
The most widely consumed traditional drinks in Burkina Faso are dolo, bandji and zoom-koom (Icard-Vernière et al., 2010). Zoom-koom is a traditional millet-based drink derived from millet or sorghum grains by lactic fermentation (Soma, 2014). It is sold and consumed in all parts of Burkina Faso by urban as well as rural people. Tapsoba et al. (2017a) showed that fermented millet zoom-koom is better than fermented sorghum zoom-koom, but unfermented sorghum zoom-koom is better than unfermented millet zoom-koom in terms of microbiological quality.
However, zoom-koom process is spontaneous, uncontrolled and usually made with varied fermentation times and temperatures, resulting in products inconsistent in quality attributes (Soma et al., 2017). In order to avoid growth of undesired microorganisms including pathogenic and spoilage microorganisms, starter cultures can be used. By the use of starter cultures, it is possible to control fermentation of such condiments, avoiding growth of pathogenic and spoilage microorganisms, leading to a product of consistent taste and quality, as well as improved marketability (Ouoba et al., 2008). In fact, Soma (2014) showed throughout a control production of millet zoomkoom using Lactobacillus fermentum strain as starter, that the lactic fermentation allowed the reduction of enterobacteria counts and kept safe the final product. Moreover, Tapsoba et al. (2017b) characterized and identified the selected LAB isolates, which could be used as starters’ cultures to improve microbiological quality and the texture of zoom-koom and then they found that Lactobacillus strains were the dominant bacteria involved in the fermentation of zoom-koom. The present study consisted to realize the formulation of liquid zoom-koom and instant flour zoom-koom using starter cultures in order to determine their impact on the nutritional, sanitary and organoleptic quality of these beverages.
Traditional production of liquid zoom-koom and instant flour zoom-koom were followed in order to establish the diagram production. Then 04 formulations (Tables 1 and 2) for each type of zoom-koom were prepared and submitted for sensorial, biochemical and microbiological analyzes. The best formulation of each type was chose for control fermentation using L. fermentum strain as starter. The final product obtained by controlled fermentation was also analyzed.
Traditional production of liquid zoom-koom and instant flour zoom-koom
Study area
The study took place in Ouagadougou, Burkina Faso. 02 artisanal producers (one producer of liquid zoom-koom located at Zogona district and another producer of instant flour zoom-koom located at Pissy district) have been selected for the study. In each production site, 03 productions were followed up during 03 weeks for the establishment of diagram production. The biological material required for zoom-koom production consists mainly of millet (Pennisetum glaucum) grains and ingredients such as tamarind (Tamarindus indica), mint (Mentha spicata L), ginger (Zingiber officinale) and sugar (Soma, 2014).
Traditional production of liquid zoom-koom
From the follow-ups, the liquid zoom-koom (Figure 1) was obtained after 10 processing steps (Icard-Vernière et al., 2010; Soma, 2014; Tapsoba et al., 2017) which are: sorting the millet grains, peeling, soaking during 13 h, washing, draining the grains, mixing with ginger (6 g/ 100 g) and mint (3 g/ 100 g), milling, filtration and sweetening (2500 g/ 5 L).
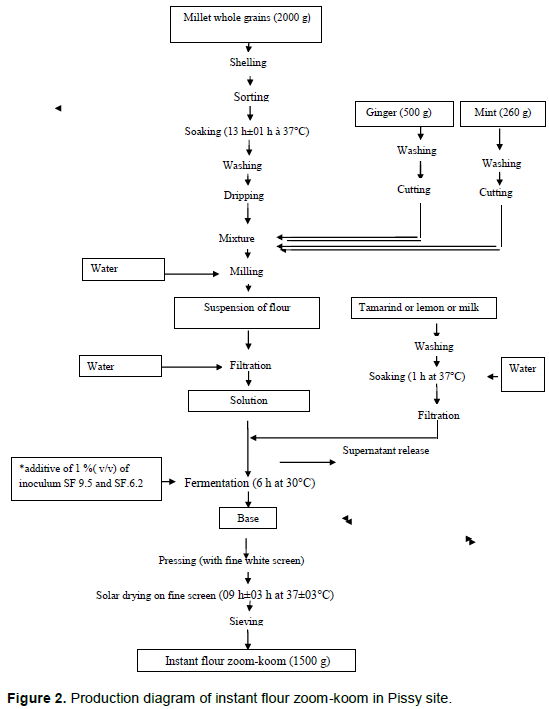
Traditional production of instant flour zoom-koom
The instant flour zoom-koom was obtained after 14 steps from the raw material to the final product (Soma, 2014): sorting the millet grains, peeling the grains, soaking, washing, draining, mixing with ginger and mint, milling, filtration, first fermentation for 06 h at 37°C, second fermentation for 12 h at 37°C, squeezing of the pellet, drying of the pellet at 40°C for 09 h, sugaring the dried paste and finally sieving.
Zoom-koom formulations
The zoom-koom formulations took place in our pilot plan named Technopole.
Liquid zoom-koom formulations
A total of 04 liquid zoom-koom formulations were prepared according to the artisanal production diagram (Figure 1). The formulations are distinguished by using separately lemon, baobab pulp, milk and tamarind. The formulations of liquid zoom-koom were summarized in Table 1.
Instant flour zoom-koom formulations
A total of 04 instant flour zoom-koom formulations were prepared according to the production diagram developed. 03 formulations were based separately on baobab pulp, tamarind and milk and the last formulation contained any baobab pulp, any tamarind and any milk. The composition of each formulation is summarized in Table 2.
Controlled fermentation in zoom-koom production Origin of L. fermentum strains used as starter culture
L. fermentum strains (SF6.2 and SF9.5) used as starter culture in zoom-koom production, were isolated from dolo and pito (sorghum beer) (Sawadogo/Lingani et al., 2007; 2008). The 2 strains were chose from 45 efficient selected strains based on their acidifying capacity, their antimicrobial activity, their capacity to produce amylases and their ability to produce exocellular polysaccharides (Sawadogo-Lingani et al., 2008).
Preparation of the inocula of L. fermentum SF9.5 and SF6.2
Each L. fermentum strain stored in glycerol at -80°C, were subcultured on MRS Agar (Liofilchem, Italy), incubated for 48 h at 37°C. The colony was transferred into 10 ml MRS broth in tube, incubated for 24 h at 37°C. Then 0.1 ml of culture broth was transferred in 10 ml MRS broth and incubated for 16-18 h at 37°C. For each strain, the culture broth was centrifuged at 8000 g for 5 min. The supernatant of each tube was removed and the pellet was washed twice in 1 ml of sterile saline (8.5 g/L NaCl and 1.5 g/L bactopeptone (Difco, France), pH 7.0). After stirring, the suspension of cells (inoculum), the concentration of viable cells was determined as described by Sawadogo-Lingani et al. (2008).
Production of liquid zoom-koom and instant flour zoom-koom by controlled fermentation using L. fermentum strains SF9.5 and SF 6.2
Liquid zoom-koom and instant flour zoom-koom were produced according to the method described in Figures 1 and 2 respectively. The inoculum of L. fermentum was used at a rate of 1% (V / V) as described by Sawadogo et al. (2008). 100 ml of inoculum, composed of 50 ml of L. fermentum SF6.2 and 50 ml of L. fermentum SF9.5, were used to inoculate separately 10 L of liquid zoom-koom and 10 L of instant flour zoom-koom to obtain a final rate of 106 cells/ml. The mixtures were incubated at 37°C.
Sampling
From the 04 formulations prepared for each type of zoom-koom, the samples were collected for biochemical (pH and acidity), microbiological (aerobic mesophilis bacteria, lactic acid bacteria, coliforms, yeasts and molds) and sensory analyzes (grading test). Sampling was also done during controlled fermentation for biochemical (pH, acidity, protein, fat, total and reducing sugar, ashes, degree brix, mineral contents), microbiological (aerobic mesophilic bacteria, lactic acid bacteria, coliforms, yeasts and molds) and sensory analyzes (difference and profile tests). Moreover during zoom-koom formulations and controlled fermentation assays, sampling was done on the raw material (millet), technological adjuvants (maize, tamarind pulp, baobab pulp, mint, ginger, sugar), millet grains during soaking (beginning and end), the liquid zoom-koom and instant flour zoom-koom.
Biochemical analyzes of liquid zoom-koom and instant-flour zoom-koom
Measurement of pH and titratable acidity
The pH of the samples was measured using an electronic pH meter (Model HI 8520, Hanna Instrument, Singapore). For the solid samples, 10 g of product were mixed with 20 ml of distilled water before pH measurement. For liquid samples, pH was measured directly in the 10 ml of beverage (Sawadogo-Lingani et al., 2007). For determination of the titratable acidity, 5 g or 5 ml of sample suspended in 50 ml ethanol (90%) were centrifuged for 5 min at 3500 g. From the supernatant, 10 ml were transferred in a flask and filled up to 50 ml with distilled water. After mixing, 10 ml of the diluted sample were titrated with 0.1 N NaOH using 1% phenolphthalein as an indicator (Sawadogo-Lingani et al., 2007). The titratable acidity (g of lactic acid per 100 ml or g of sample) was calculated according to Amoa-Awua et al. (1996).
Determination of protein content
Crude proteins contents were determined from the total nitrogen assay according to the Kjeldahl method NF V 03-50 (1970). The procedure comprises a mineralization, a distillation and a titration by sulfuric acid.
Determination of fat content
The fat content of the samples was determined by Soxhlet extraction according to the international standard (ISO-659, 1998). The extraction was carried out with hexane. The fat content was determined by weighing after evaporation of the hexane by distillation.
Determination of total and reducing sugar contents
Total sugar content has been estimated according to Montreuil and Spik (1963). 0.2 g of each sample was weighed and diluted in a flask containing 200 ml of distilled water. Then, 2 ml of orcinol solution and 7 ml of sulphuric acid solution 60% were added to 1 ml of the obtained solution. The mixtures were boiled for 20 min and placed in a dark place during 45 min. The reading of the optic density was done by a spectrophotometer (PG Instruments, England) at 510 nm. The standardization curve has been achieved using glucose (0.5 mg/ml) as reference. ¶For reducing sugars, the quantification was done according to the method of Bernfeld (1955). The previous extract was introduced into a test tube; 0.5 ml of distilled water and 0.5 ml of DNS (Di-Nitro Sulfate) solution were added. The mixture was heated in a water bath at 90°C for 5 min. After cooling, 2 ml of distilled water were added and then the absorbance of the solution was read using a spectrophotometer (PG Instruments, England) at 490 nm. The standardization curve has been achieved using glucose (0.5 mg/ml) as reference.
Ashes contents determination
The ashes content was determined according to the standard V03-760 (1981). In three crucibles, 3 g of zoom-koom samples were placed in each one. The crucibles were submitted to a mineralization in the oven (Nabertherm) at 650°C during the night (16 - 18 h). After this time, the crucibles were withdrawn, cooled in the desiccator during 60 min before being weighed. The operation has been renewed until obtaining a constant weight.
Measurement of degree brix (total soluble matter)
The degree Brix was measured using a refractometer (Euromex, Holland). ¶A drop of liquid zoom-koom was deposited on the dial of the apparatus. ¶The reading was done through the luminous indicator of the apparatus.
Mineral composition: P, Mg, Ca, Fe, Zn
Determination of phosphorus
(i) After mineralization of the organic matter with a mixture of perchloric acid (HClO4) and oxygenated water (H2O2), the phosphorus of the sample was in the form of orthophosphate (H3PO4), which in acid medium combines with molybdate to give a phosphomolybdic complex H3P(MO3O10)4. This complex in the presence of ascorbic acid was reduced to molybdenum blue, which allows a colorimetric determination (the intensity of the coloration depends on the phosphate content).
(ii) In a clean, and dry mattress, 1 g of sample, 4 mL of H2O2 and 2 mL of HClO4 were introduced. The mineralization was carried out by gradual heating of 15-20 min at 50, 70, 90, 100 and 200°C on the heating plates until obtaining a yellowish or whitish solution with the release of whitish smoke. After cooling, the volume of mineralization was supplemented to 100 mL with distilled water.
(iii) In a tube test, 1 ml of the digestion product filtrate, 5 ml of distilled water, 4 ml of a solution composed of 10 ml of H2SO4 (6N), 10 ml of 2.5% ammonium molybdate and 10 ml of 10% ascorbic acid were introduced. The coloring was allowed to develop for 30 min and then the absorbance was read at 820 nm using the spectrophotometer. The phosphorus content was determined using a standard curve made from a stock solution of K2HPO4 at 20 mg / ml.
Determination of Magnesium, Calcium, Zinc and Iron contents
After wet mineralization, the different ions were assayed by atomic absorption. About 1 g of dried sample was digested in a 50 ml mixture with 4 ml of a mixed solution composed of perchloric acid (HClO 4) 60% and concentrated sulfuric acid (H2SO4) (7/1: V / V) and 15 ml concentrated nitric acid (HNO3). Heating was gradual up to 345°C. After complete digestion, the mineralization was cooled; the volume was reduced to 50 ml after filtration. For the determination of calcium and magnesium, to 0.2 ml of filtrate, 4.8 ml of an aqueous solution of lanthanum (La2O3) 1% were added. The addition of lanthanum eliminated interferences of phosphorus and aluminum. The iron was diluted with distilled water. The atomic absorption spectrophotometer (Perkin-Elmer model 303) was used to read the absorbance at the following wavelengths: 422.7 nm for Ca, 285.2 nm for Mg, 248 nm for Fe and 213.9 for Zn. The contents were determined using standard curve for each element.
Microbiological analyzes of the samples
For the preparation of stock solutions, there were tenfold dilutions and inoculation in agar plates. For all the determinations, 10 g of the samples were homogenized in a stomacher with 90 ml of sterile peptoned buffered water. Tenfold serial dilution was prepared and spread-plated for microorganisms count. 1 ml of suitable diluted was use for spreading. Aerobic mesophilic bacteria (AMB) were enumerated in plates of Plate Count Agar (Liofilchem, Italy) incubated at 30°C for 72 h (ISO 4833, 2003).
Yeasts and Moulds were counted by cultivation on Yeast extract Glucose-Chloramphenicol Agar (Oxoid, England) after incubation at 25°C for 4-5 days according to ISO 7954 (1988) standard. Lactic acid bacteria (LAB) were counted by cultivation on De Man, Rogosa and Sharpe Agar (Liofilchem, Italy) incubated anaerobically in an anaerobic jar at 37°C, for 3 days according to ISO 15214 (1998) standard. Coliforms were enumerated on Violet Red Bile Agar (VRBA) (Liofilchem, Italy), incubated at 37°C (Total Coliforms) or 44°C (Thermotolerant coliforms) for 24 h. The total and thermotolerant coliforms were enumerated according to ISO 4832 (2006) and NF V08-060 (2009) respectively.
Sensory analysis of liquid zoom-koom and instant flour zoom-koom samples
Grading test on the formulation samples of liquid zoom-koom and instant flour zoom-koom
The purpose of this test was to classify the 04 formulations of liquid zoom-koom as well as instant flour zoom-koom according to the tasters preference. 40 tasters were asked to give their opinion on the samples. The formulation that received the highest rate was retained for controlled fermentation with L. fermentum starter culture.
Difference test on liquid zoom-koom and instant flour zoom-koom produced with L. fermentum strains SF9.5 AND SF6.2
The selected formulation retained during grading test was reproduced by using L. fermentum strains SF9.5 and SF6.2. This test aims to compare 02 liquid zoom-koom samples (one was produced with the L. fermentum strain as ferment and the second was prepared without L. fermentum and serves as a control); it aims also to compare 02 instant flour zoom-koom samples (one was produced with the L. fermentum strain as ferment and the second was prepared without L. fermentum and serves as a control). This difference test required a panel of 24 tasters as describes by Cochran and Cox (1957).
Profile test on liquid zoom-koom and instant flour zoom-koom produced using L. fermentum strains SF9.5 AND SF6.2
The aim of this test was to determine the preference of the panelists by comparing the acidity, the sweet taste and the spicy taste of liquid zoom-koom and instant flour of zoom-koom obtained with starter cultures. This test required 06 experienced panelists.
Statistical analysis
All data were subjected to ANOVA with XLSTAT-Pro statistical software 7.5.2 and the means were compared using the Student Newman-keuls test at the probability level p˂0.05. The curves were obtained using Microsoft Excel 2010.
Biochemical, microbiological and sensorial characteristics of zoom-koom formulations
Acidity and pH
The pH of the liquid zoom-koom samples varied between 5.02 and 5.13 for a titratable acidity which varied between 1.21 and 1.89 g of lactic acid / 100 g of product (Table 6). The pH of instant flour zoom-koom varied between 5.11 and 5.70. The acidity varied between 0.81 and 1.21 g of lactic acid / 100 g of product (Table 7). These values showed that both types of zoom-koom are acidic beverages (pH<6). The acidification of the foodstuffs was a method used to stabilize the products. Many studies (Thompson and Weber, 1979; ¶Champagne and Phillippy, 1989) reported the effect of acidification of food by ¶improving the absorption of certain minerals.
Microbiological quality of liquid zoom-koom and instant flour zoom-koom formulations
The aerobic mesophilic bacteria counts of the 04 formulations of instant flour zoom-koom varied between 3.9×107cfu / g and 8.9×108 cfu / g, and the lactic acid bacteria counts between 1.8×107cfu / g and 3.0×108 cfu / g (Table 7). For total coliforms, their charge varied between 10 cfu / g and 4.7×104cfu / g. The thermotolerant coliforms ranged from 10 to 1.5×104 cfu / g. The yeasts and molds counts varied from 10 to 2.0×105cfu / g. No significant difference was observed (p<0.05). Based on aerobic mesophilic bacteria results, the 04 formulations of instant flour zoom-koom were unsatisfactory according to the recommendations by CECMA (2009) which limit is 106 cfu/g. Instant flour zoom-koom incorporated separately with milk and baobab pulp were conforms to the values recommended by CECMA (2009) which limited thermotolerant coliform to 102 cfu/g, but instant flour zoom-koom incorporated separately with tamarind. According to the standards on dried foods recommended by JORA (2009) who limited total coliform to 102 cfu/g, only instant flour zoom-koom incorporated with baobab pulp was conform. The yeasts and molds concentration in instant flour zoom-koom incorporated separately with baobab pulp, tamarind and traditional zoomkoom were found to be conforms to the recommendations by MSL (2018) (limit: 104 cfu/g).
For the 04 liquid zoom-koom formulations, the aerobic mesophilic bacteria varied between 1.5×107cfu / ml and 3.3×108cfu / ml (Table 6). The lactic acid bacteria varied between 9.0 × 10 6 and 1.7 × 10 8cfu / ml. The total coliforms ranged from 2.0 × 10 2 to 5.2 × 10 3 cfu / ml. Thermotolerant coliforms ranged from 10 cfu / ml to 2.3×103 cfu / ml. The yeasts and molds counts were 10 cfu / ml and 3.0×103 cfu/ ml. No significant difference was observed (p<0.05). The aerobic mesophilic bacteria as well as total coliforms concentrations in the 04 formulations (liquid zoom-koom incorporated separately with lemon, milk, baobab pulp and tamarind) were found to be non conforms to the recommendations by CECMA (2009) (limits: 106 cfu/ml for aerobic mesophilic bacteria and 10 cfu/ml for total coliforms). About thermotolerant coliform value, only liquid zoom-koom incorporated with lemon was conform to the recommendations by CECMA(2009) (limits: 102 cfu/mL). The yeasts and molds concentrations in the 04 formulations (liquid zoom-koom incorporated separately with lemon, milk, baobab pulp and tamarind) were found to be conforms to the recommendations by CECMA (2009) (limits: 104 cfu/ml).
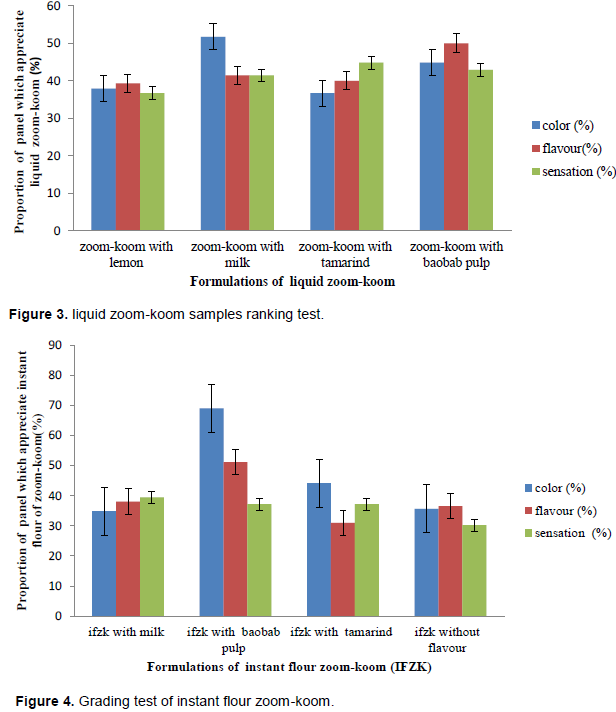
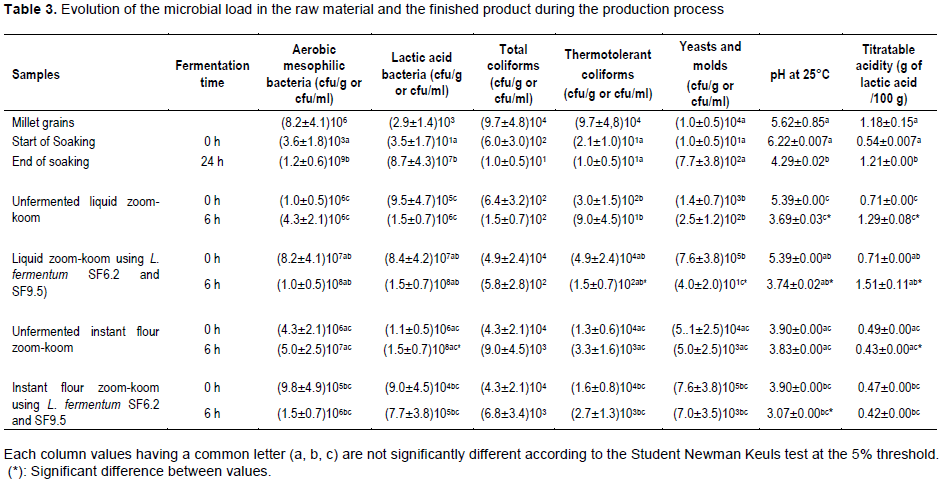
GRADING TEST
The results of the ranking test of the 04 liquid zoom-koom and the 04 instant flour zoom-koom formulations were showed in Figures 3 and 4. Concerning the color of liquid zoom-koom, 51.7, 44.8, 37.9 and 36.7 % of the panelists liked zoomkoom incorporated separately with milk, baobab pulp, lemon and tamarind respectively. As for the flavor, 50, 41.4 and 40% of the panelists expressed a good appreciation on the liquid zoom-koom incorporated separately with baobab pulp, milk and tamarind respectively. The mouth feel (sensation of tasting) test showed a good appreciation of liquid zoom-koom incorporated with tamarind (44.8% of the panel), baobab pulp (42.9% of the panel), milk (41.4% of the panel) and lemon (36.7% of the panel). From these values, it appeared that the liquid zoom-koom with milk presented the best color; the liquid zoom-koom with baobab pulp presented the best aroma and the liquid zoom-koom with tamarind had the best mouth feel. 69, 44.2 and 34.9% of the panelists liked the color of instant flour zoom-koom incorporated separately with baobab pulp, tamarind and milk respectively whereas 35.7% of the panel enjoyed traditional instant flour zoom-koom. For the flavor or aroma of the instant flour zoom-koom incorporated with baobab pulp, tamarind and milk was well appreciated by 51.2, 31 and 38.1% respectively; 36.6% liked the aroma of the traditional instant flour zoom-koom. According to the sensation after tasting (mouth feel), the instant flour zoom-koom incorporated separately with baobab pulp and tamarind were found to be pleasant by 37.2% of the panelists, whereas the instant flour zoom-koom incorporated with milk and traditional instant flour zoom-koom were judged pleasant by 39.5 and 30.2% respectively. So, it appears that the instant flour zoom-koom incorporated with baobab pulp showed the best color and aroma and the instant flour zoom-koom incorporated with milk had the best month feel.
In sum, the liquid zoom-koom and instant flour zoom-koom incorporated with the baobab pulp were found to have the best organoleptic characteristics; so theses formulations were selected for controlled fermentation using L. fermentum strains.
Biochemical, microbiological and sensorial characteristics of zoom-koom PRODUCED BY controlled fermentation using L. fermentum strains (SF9.5 AND SF 6.2) As Starter Culture
Biochemical characteristics
pH and titratable acidity: The values of pH and titratable acidity of the zoom-koom samples (p< 0.05) obtained by controlled fermentation are presented on Table 3. The pH of the liquid zoom-koom samples (Table 3) varied from 3.69 to 5.39. The titratable acidity was between 0.71 and 1.51 g of lactic acid for 100 g of product. For instant flour zoom-koom samples, the titratable acidity varied from 0.42 to 0.49 g of lactic acid for 100 g of product. The pH value ranged from 3.07 to 3.9. These results showed that the traditional and controlled processing permitted to have an acid beverage. The lowering of the pH and the increase in acidity result from a lactic fermentation (Sawadogo-Lingani et al., 2010).
Proteins contents
The proteins contents of controlled fermented zoom-koom samples were respectively 25.83% dry matter (d.m) for the liquid zoom-koom without L. fermentum, 28.41% for the liquid zoom-koom with L. fermentum, 4.57% for the instant flour zoom-koom without L. fermentum and 3.76% for the instant flour zoom-koom with L. fermentum (Table 4). These values showed that the liquid zoom-koom fermented by L. fermentum is richer in protein than the liquid zoom-koom without L. fermentum. On the other hand the instant flour zoom-koom without L. fermentum gave a content of protein higher than the instant flour zoom-koom fermented by L. fermentum.
Tapsoba and collaborators in 2017 obtained a protein content in the liquid zoom-koom fermented by W. confusa/cibaria and without W. confusa/cibaria ranging between 17.66 and 25.30% d.m respectively. The protein content of our samples was slightly higher than those reported by Tapsoba et al. (2017). The essential function of a food protein was to satisfy the needs for the nitrogen organization and essential amino acids. The quality of a protein was related primarily to its composition in essential amino acids.
Lipids contents
The fat contents of zoom-koom samples were 6.48% d.m (dry matter) and 5.04% d.m respectively for the liquid zoom-koom without L. fermentum and the liquid zoom-koom fermented by L. fermentum (Table 4). The contents were 2.62 and 4.62% d.m respectively for the instant flour zoom-koom without L. fermentum and the instant flour zoom-koom fermented by L. fermentum. These values showed that the fat content of the liquid zoom-koom without L. fermentum was higher than the fat content of the liquid zoom-koom fermented by L. fermentum. On the other hand the instant flour zoom-koom without L. fermentum contained less fat than the instant flour zoom-koom fermented by L. fermentum. Tapsoba et al. (2017), obtained fat contents of liquid zoom-koom without W. cibaria/confusa and with W. cibaria/confusa were 4.74 and 5.55% d.m respectively. The fat contents of our samples are similar to those obtained by Tapsoba and collaborators in 2017. A degradation of the lipids could cause the appearance of free fatty acids metabolized in aromatic compounds such as alcohols, ketones and aldehydes. The lipolysis leads to a development of the flavor. Several lactic acid bacteria isolated from the dairy products, mainly Lactobacillus spp presented a lipolytic activity (Montanari et al., 2013).
Total sugar and reducing sugars contents
The total sugar contents were 65.74% d.m (dry matter) and 64.83% d.m respectively for the liquid zoom-koom unfermented and the liquid zoom-koom fermented by L. fermentum (Table 4).Total sugar contents were 92.63 and 91.43% d.m respectively for the instant flour of zoom-koom unfermented and the instant flour zoom-koom fermented by L. fermentum. For the reducing sugars, the contents were 32.1 and 36.9% dm respectively for the liquid zoom-koom unfermented and the liquid zoom-koom fermented by L. fermentum. The contents were 29.88 and 47.43% dm respectively for instant flour zoom-koom unfermented and instant flour zoom-koom fermented by L. fermentum. In contrast, samples (liquid zoom-koom, instant flour zoom-koom) fermented using L. fermentum contained more reducing sugars than those unfermented. Tapsoba and collaborators in 2017 obtained total sugar contents in samples of liquid zoom-koom unfermented and liquid zoom-koom fermented by L. fermentum were 68.86 and 76.24% d.m respectively. The total sugar contents of our samples were lower than those obtained by Tapsoba et al. (2017). This difference (p<0.05) could be explained by the quantity of sugar added during the processing of zoom-koom, varying according to the processing. The first stage of food fermentation was the use of the glucids by the LAB. The LAB was able to degrade a broad range of oses like lactose and galactose, but also saccharose, maltose, glucose, fructose and α-galactosides. The major metabolite of this degradation was the lactic acid. Some few LAB like L. fermentum, Lb. sanfranciscensis and Lb. pontis were able to use the fructose as carbon source (Stolz et al., 1995).
Ash contents
The ash contents were 1.72 and 1.95% d.m respectively for the liquid zoom-koom fermented by L. fermentum and the unfermented liquid zoom-koom (Table 4). The values were lower for the unfermented instant flour zoom-koom (0.18% m.s) and the instant flour zoom-koom fermented by L. fermentum (0.19% d.m).The liquid zoom-koom contained more minerals than the instant flour of zoom-koom. Tapsoba and collaborators in 2017 found 0.32 and 0.69% d.m as ashes contents for unfermented liquid zoom-koom and liquid zoom-koom fermented by L. fermentum respectively. Our samples of liquid zoom-koom contained more ashes (1.72-1.95% d.m) than the samples analyzed by Tapsoba et al. (2017)(0.18-0.19% d.m). The difference observed could be explained by the effect of dilution and the manufacturing process.
Brix
The total soluble matter content (°Brix) of the samples of unfermented liquid zoom-koom and zoom-koom fermented by L. fermentum was 15 and 16.5% respectively (Table 4). Tapsoba et al. (2017) obtained 18% representing a mean value of total dried matter in unfermented liquid zoom-koom and fermented zoom-koom; this value was higher compared to the total soluble dried matter found in our samples of liquid zoom-koom.
Minerals contents of zoom-koom samples
The minerals contents of liquid zoom-koom and instant flour zoom-koom fermented using L. fermentum on the one hand and unfermented liquid zoom-koom and instant flour zoom-koom on the other hand were showed in Table 5. The calcium contents varied between 0.218 and 0.435 g / 100 g d.m. The magnesium content varied between 0.039 and 0.065 g / 100 g d.m. The iron content was between 2.50 and 3.08 mg / 100 g. The phosphorus content was between 0.010 g and 0.012 g / 100 g d.m. From these results the liquid zoom-koom fermented using L. fermentum contained more calcium, magnesium and iron compared to the unfermented liquid zoom-koom. Similarly, instant flour zoom-koom fermented using L. fermentum contained more iron and phosphorus compared to unfermented instant flour zoom-koom. The micro-organisms played a role in the synthesis of vitamin factors, in the absorption of calcium, magnesium and iron. Several metals such as Fe2+, Fe3+, Mg2+, Mn2+ and Zn2+ were necessary to the growth of the lactic bacteria (Salonen et al., 2014; Walsh et al., 2014).
Microbiological characteristics of controlled fermented zoom-koom
After 24 h of soaking the millet grains in water (Table 3), the concentration of aerobic mesophilic bacteria reached 1.2×109cfu / g. The soaking of the grains was always accompanied by a lactic fermentation, the drop in pH from 6.22 to 4.29 and the increase in titratable acidity (0.54 to 1.21 g of lactic acid / 100 ml); as well as the development of lactic acid bacteria whose concentration increased from 3.5×10 1 cfu / g (beginning of soaking) to 8.7 × 10 7 cfu / g at the end of soaking. During the same period, there was a drop of pH as well as a decrease of total coliforms from (6.0×102 cfu / g to less than 10 cfu / g) and thermotolerant (2.1×102 cfu / g to less than 10 cfu / g). This phenomenon was could be related to the acidification of the environment caused by the development of lactic acid bacteria.
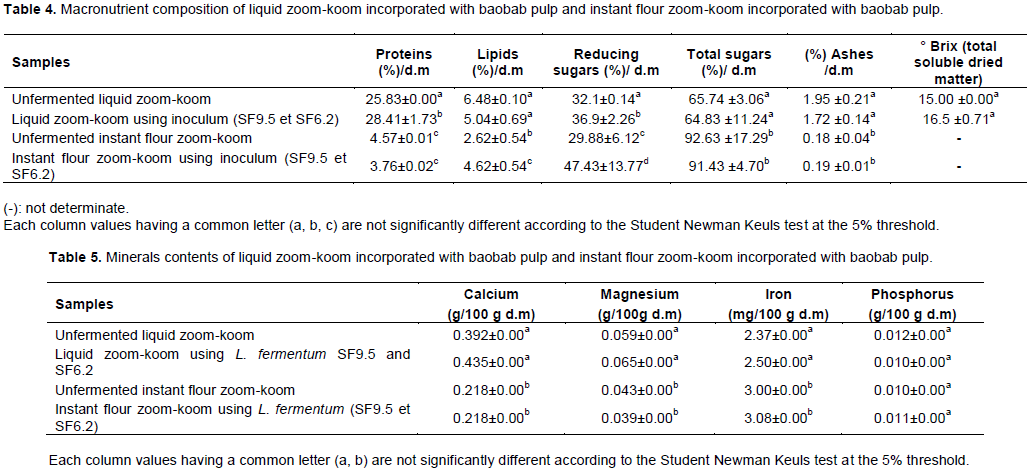
Following the fermentation process for 06 h, microorganisms were counted at the beginning (0h) and the end (6 h) of fermentation. The aerobic mesophilic bacteria of the liquid zoom-koom samples varied from 1.0 × 10 6 ufc / ml to 1.0 × 108cfu / ml (Table 3). The lactic acid bacteria varied from 9.5×105 cfu/ml to 1.5×108 ufc/ml. Total coliforms ranged from 1.5×10² cfu/ml to 4.9×104 cfu/ml. The thermotolerant coliforms varied from 9.0× 101 cfu/ml to 4.9 × 104 cfu/ml. The yeasts and molds ranged from 4.0 × 101 cfu / ml to 7.6 × 10 4 cfu/ml.
The comparison of spontaneously fermented samples and the samples obtained by controlled fermentation using L. fermentum strains SF6.2 and SF9.5, in 06 h of fermentation revealed that natural fermentation reduced total coliforms by 76.56 % in 06 h, 70% for thermotolerant coliforms and by 82.14% for molds. In the same time, the controlled fermentation with L. fermentum SF6.2 and SF9.5 strains, reduced total coliforms by 98.82%, 99.69% for thermotolerant coliforms and by 99.69 % for molds. For instant flour zoom-koom, the microorganisms counts ranged from 9.8×105 cfu/g and 5.0×107 cfu/g (aerobic mesophilic bacteria), from 9.0×104 cfu/g to 1.5×108 cfu/g (lactic acid bacteria), from 6.8×103 cfu/g to 4.3×104 cfu/g (total coliforms), from 2.7×103 cfu / g to 1.6×104 cfu / g (thermotolerant coliforms), from 5.0×10 3 cfu/g to 7.6×105 cfu/g (yeasts and molds). We observed that natural fermentation reduced total coliforms by 79.07 and 74.61% for thermotolerant coliforms and by 90.2% for molds. The controlled fermentation of the instant flour zoom-koom using L. fermentum strains (SF6.2 and SF9.5) caused a reduction of 84.19% total coliforms, 83.12% thermotolerant coliforms and 46.15% molds. The effect of starter culture (L. fermentum SF6.2 and SF9.5) was less effective on the molds. The selected strains of L. fermentum used as starter cultures for zoom-koom production seem to express their antimicrobial capacity against coliforms, and yeasts and molds during fermentation.
The selected strains of L. fermentum SF6.2 and SF9.5 confirmed their acidifying capacity and their ability to inhibit the pathogens on the base of which they had been selected (Sawadogo-Lingani et al., 2008). The use of these strains as starter cultures in the zoom-koom technology could improve the sanitary quality in order to assure consumers safety. Previous studies showed the presence of coliforms at high levels in the zoom-koom produced and sold in Ouagadougou city (Barro et al., 2002; Bsadjo-Tchamba et al, 2014) and similar beverages like kunun-zaki, a traditional millet-based drink from Nigeria (Gaffa, 2002; Elmahmood and Doughari 2007). In addition, zoom-koom processing in Burkina Faso does not include a pasteurization step to assure the safety of the products (Soma et al., 2017; Tapsoba et al., 2017). This study revealed that the 02 major ingredients that bring high levels of micro-organisms (pathogenic bacteria, lactic acid bacteria, yeasts and molds) were mint and ginger. The lactic fermentation could contribute to reduce pathogenic bacteria after 24 h of fermentation. Tapsoba et al. (2017) revealed that starters could considerably improve the hygienic quality of the zoom-koom.
The concentrations of aerobic mesophilic bacteria, total coliforms in unfermented liquid zoom-koom and instant flour zoom-koom on the one hand and in fermented liquid zoom-koom and fermented instant flour zoom-koom were found to be non conforms according the recommendations by CECMA (2009). However the concentrations of yeasts and molds in all the samples of zoomkoom were found to be conforms to the recommendations by CECMA (2009).
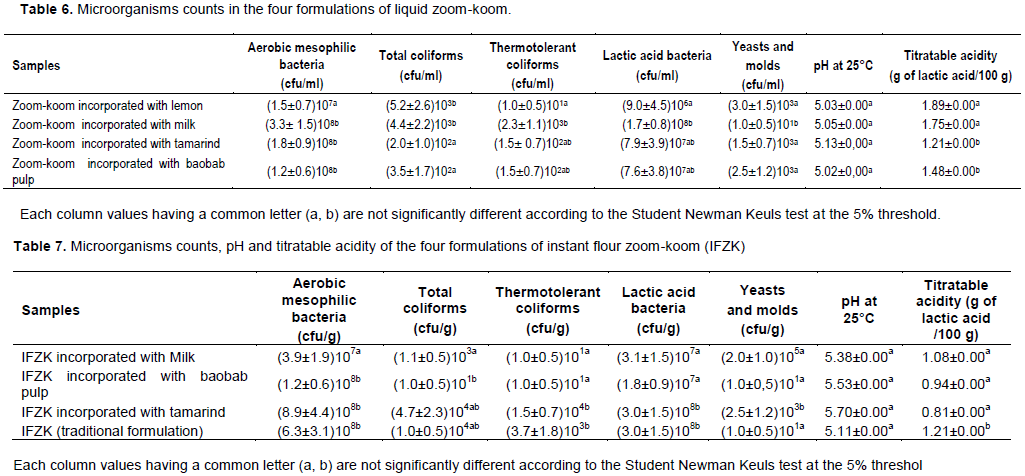
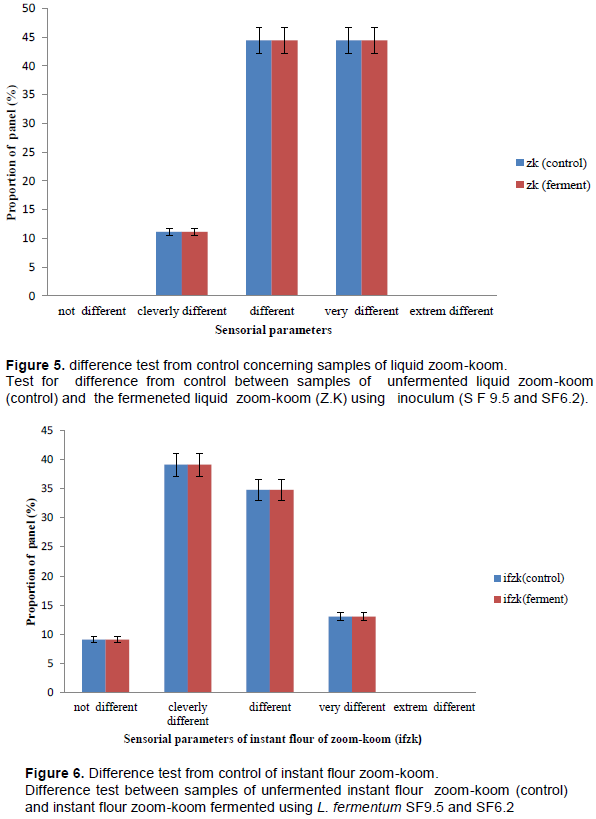
Sensorial characteristics of controlled fermented zoom-koom
Difference test: The results of the difference test were represented in Figures 5 and 6. From the difference test between the liquid zoom-koom obtained by natural or spontaneous fermentation used as control and the liquid zoom-koom fermented using L. fermentum strains (SF9. 5 and SF6.2), 11.11% of the tasters observed a slight difference between these two types of liquid zoom-koom and 44.44% of the panel observed a clear difference between these two types of liquid zoom-koom. From the difference- test between the instant flour zoom-koom (control) and fermented instant flour zoomkoom, 9.1% of the tasters did not notice any difference between these two types of zoom-koom. On the other hand, 39.13 and 34.78% of the panelists observed a slight and clear difference between these two types of instant flour zoom-koom; 13.04% of the panel observed a clear difference between these 02 types of instant flour zoom-koom. The results obtained in the present study were comparable to those observed with kunun zaki in Nigeria by Gaffa and Ayo in 2002. Indeed, Gaffa and Ayo (2002) proved that the preparation of kunun zaki (beverage similar to zoom-koom) with starter cultures improved the organoleptic quality of the product.
Profile test: The results from profile test were shown in Figure 7. Concerning the liquid zoom-koom processed by controlled fermentation using L. fermentum (SF9.5 and SF6.2), 70% of the panelists appreciated its sweetened taste, 90% appreciated its acidulated taste and 80% appreciated its spicy taste. For instant flour zoom-koom processed by controlled fermentation using L. fermentum (SF9.5 and SF6.2), 67% of the panel appreciated its sweet taste, 83% its acidulated taste and 100% its spicy taste. These results showed that the liquid zoom-koom fermented with the selected L. fermentum (SF9.5 and SF6.2), had a sweet and acidulated taste more appreciated than the fermented instant flour zoom-koom. On the other hand the fermented instant zoom-koom flour would have a more pungent taste more appreciated than the fermented liquid zoom-koom.