ABSTRACT
Bacterial leaf blight (BLB) of rice is one of the most destructive diseases affecting rice fields. Xanthomonas oryzae pv. oryzae (Xoo) is the causal agent of BLB. Two BLB resistance genes, Xa21 and Xa7, were transferred into the susceptible indica cultivar Bacthom7 (BT7) by using marker-assisted selection with markers pTA248 for Xa21 and ID7 for Xa7. Improved BT7 lines carrying the two resistance genes were inoculated with three isolates of the Xoo from Northern Vietnam and evaluated for agronomic traits. Artificial inoculation of 13 lines with three Xoo races identified nine highly resistant lines with wide-spectrum resistance to Xoo, including D1, D2, D3, D6, D7, D8, D9, D10 and D12. These lines were similar to recurrent parent BT7 with regard to external appearance, yield performance and grain quality. On the basis of agronomic traits and the level of resistance to BLB, two promising lines, D6 and D9 were further selected. These two lines could efficiently contribute to rice production for food security and food safety in northern Vietnam.
Key words: Xanthomonas oryzae pv. oryzae, resistance genes, near-isogenic lines, marker-assisted selection (MAS), improved rice lines.
Bacterial leaf blight (BLB) caused by Xanthomonas oryzae pv. oryzae (Xoo) is one of the most devastating diseases in rice fields in Asia. The disease has recently become more serious in northern Vietnam because it
now appears during two crop seasons, especially among hybrid rice varieties. BLB caused yield decreases of up to 60% (Mew et al., 1982). Thousands of hectares of cultivated land are affected by BLB annually in India, with yield losses amounting up to 60% (Sirivastava, 1972). BLB spreads widely during the summer season in coastal areas of northern Vietnam (Plant Protection Department, MARD). Areas affected by BLB increased by 30 to 70% in 2012. Although BLB can occur at any stage and in any organ of rice plants, infection particularly reduces yield at the booting, heading and milk stages. However, practical chemical methods that can be applied to control BLB remain to be established. Therefore, deployment of resistant varieties and integrated pest management are important solutions to controlling BLB. Thus far, a total of 38 BLB-resistant genes have been identified in rice (Khan et al., 2014). Among them, four BLB-resistant genes were mapped on rice chromosome 4 (Xa1, Xa2, Xa14 and Xa25), one on chromosome 5 (xa5), one on chromosome 6 (Xa7), one on chromosome 8 (xa13) and six on chromosome 11 (Xa3, Xa4, Xa10, Xa21, Xa22 and Xa23). The locations of the remaining BLB-resistant genes are still ambiguous.
Near-isogenic lines (NILs) and pyramided lines (PYLs) that are almost identical to parental lines, except target genes, are very useful genetic resources for genetic improvements to rice. The NILs can be used to introduce a target gene into improved rice cultivars without inducing any adverse effects such as sterility or unfavorable linkage drags such as tall plant height (Yara et al., 2010). However, the pathogen can evolve to overcome a resistant cultivar that carries a single resistance gene after large-scale and long-term cultivation. Xa4 showed resistance to BLB in the Philippines in the 1970s, but this was later overcome (Mew et al., 1992). Recently, Xa21 caused a reduction in the resistance level of Xoo races in the Philippines, India, Korea and China (Lee et al., 1999; Marella et al., 2001; Xu et al., 2012). In contrast, PYLs that carry more than two BLB resistance genes showed more durability and a higher level of resistance to BLB than lines carrying a single resistance gene (Pradhan et al., 2015). PYLs can delay the emergence of virulent Xoo races against BLB resistance genes. However, pyramided resistance genes, which show similar reactions to BLB, are difficult to develop through conven-tional breeding methods. Marker-assisted selection (MAS) has unique advantages to overcome this limitation because MAS relies on DNA polymorphism rather than phenotypic selection (Collard and Mackill, 2008).
Bacthom7 (BT7) is a high-quality cultivar and is widely cultivated in northern Vietnam but is also susceptible to Xoo. Among the reported resistance genes, Xa7 and Xa21 showed wide-spectrum resistance in Asia (Vera Cruz et al., 2000; Webb et al., 2010). Thus, one of the promising strategies to effectively improve the resistance level of BT7 is pyramiding these two resistance genes. Previously, a BT7-carrying BLB resistance gene Xa21 (BT7-Xa21) was developed and released as a new variety, BT7KBL.
Therefore, the objective of the present study was to improve BLB resistance by pyramiding two resistance genes, Xa7 and Xa21 into indica cultivar Bacthom7 (BT7). In this study, MAS was applied to improve accuracy as well as efficiency of gene pyramiding.
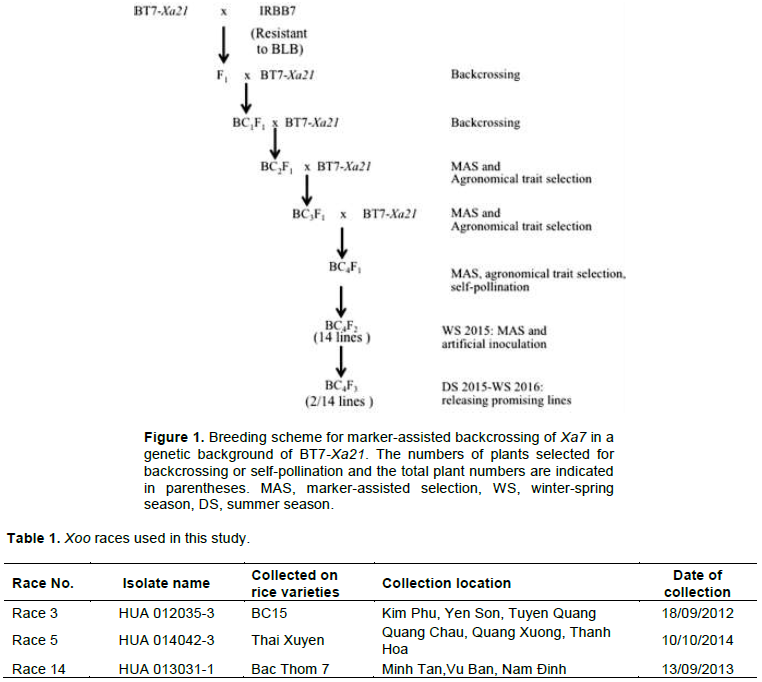
BT7-Xa21, which carries BLB resistance gene Xa21 in a genetic background of BT7, was used as recurrent parent. One IR24 NIL, IRBB7 was used as donor parent for Xa7 and IR24 was used as a susceptible control in BLB resistance evaluation. The materials were planted at Vietnam National University of Agriculture, Hanoi, Vietnam. To produce F1 plants, BT7-Xa21 was crossed with IRBB7 (Figure 1). The F1 plants were backcrossed with BT7-Xa21. In the BC1F1 generation, MAS was used to select plants with resistance alleles of Xa21 and Xa7. A similar strategy was applied until the BC4F2 generation. The BC4F2 plants were then self-pollinated to produce a BC4F3 generation. Finally, 13 BC4F3 lines carrying the two BLB resistance genes Xa21 and Xa7 were inoculated with 3 Xoo races and evaluated for agronomic traits.
Isolation of Xoo strains and evaluation of BLB resistance level
BLB-infected rice leaf samples were collected in farmers’ fields in Tuyen Quang, Nam Dinh, and Thanh Hoa provinces from 2012 to 2014 (Table 1). The isolation, culture and artificial infection was done following Furuya et al. (2012). The infected leaf was cut into 1-cm-long specimens and sterilized with 70% ethanol followed by 1% H2O2 solution. Each sample was soaked in 1 ml of distilled water and the solution was streaked on Wakimoto medium. To develop bacterial colonies, the culture was kept on a bench at room temperature for 4 days.
Yellow bacterial colonies were picked and transferred to a new clean Wakimoto medium and further cultured for 2 days. The cultured Xoo colonies were diluted to about 109 cfu/ml for artificial inoculation. Plant inoculation was carried out by clipping the tip of leaf (about 2 to 3 cm) with scissors that were dipped into the bacterial solution. The lesion lengths (cm) on the inoculated leaves were measured at 18 days after inoculation. The level of resistance was categorized as follows: lesion length <4.0 cm was highly resistant (HR), 4.0 to 8.0 cm was resistant (R), 8.0 to 12.0 cm was moderately resistant (MR), and >12.0 cm was susceptible (S).
DNA isolation and marker-assisted selection
Marker-assisted backcross was conducted to select plants that carried Xa7 and Xa21. At the BC1F1 generation, plants homozygous for Xa21 and heterozygous for Xa7 were selected. ID7 marker (forward 5′-ATA TTC ACC AAA TCA TTC CCT C-3′, reverse 5′-ATA CAA CCC TAA ACC CAT CTC A-3′) was applied to select plants that carried Xa7 (Zhang et al., 2009). pTA248 markers (forward 5′-AGA CGC GGA AGG GTG GTT CCC GGA-3′, reverse 5′-AGA CGC GGT AAT CGA AAG ATG AAA-3′) linked to Xa21 were used to select the plants that carried Xa21 (Williams et al., 1992).
Leaves (1.0 to 2.0 cm long) were harvested at mature or young stages and stored in a deep freezer for long-term storage or a refrigerator for short-term storage until use. Two DNA extraction methods were used: the CTAB method (Varghese et al., 1997) or the TPS method (Monna et al., 2002). The extracted DNA was dissolved into half strength of TE and diluted to 50% with H2O just before PCR preparation. PCR was conducted in Gene Atlas (Astes, Fukuora, Japan). The PCR reaction mixture (10 μl) contained 5 μl of Dream Taq Green PCR Master mix (Thermo Scientific, Waltham, MA, USA), 0.15 μl of primers (0.3 μM each), 2 μl DNA solution and 2.7 μl H2O. The thermal cycler was programmed as follows: initial denaturation for 2 min at 95°C (pTA248) or 5 min at 95°C (ID7); followed by 35 cycles of 95°C for 30 s, 50°C for 30 s, and 72°C for 1 min; and a final extension at 72°C for 5 min. The PCR products were separated in 1% agarose gels (pTA248) or 2% (ID7) by electrophoresis at 100 V for 45 min in TAE buffer. Gels were stained in ethidium bromide solution and then photographed under ultraviolet light.
Evaluation of agronomic traits
Rice lines were evaluated in the field at Vietnam National University of Agriculture, Hanoi, Vietnam during the spring season (January to June) in 2016. Plants were numbered and grown in numerical order in nursery beds that were 5 m in length with row spacing of 20 cm and plants were spaced 20 cm apart. Seven agronomic traits were evaluated in the BC4F3 individuals that were homozygous for Xa7 and Xa21. The traits investigated comprised days to heading (DH), plant height (PH), panicle length (PL), number of spikelets per panicle (NSP), number of grains (NG), number of panicles per plant (NPP) and 1000-grain weight (TGW) (Huang et al., 2012; Yara et al., 2010). Aromatic testing was performed according to the method described by Kibria et al. (2008). Briefly, 40 brown rice seeds were placed in a test tube and 5 ml of 1.7% (v/v) KOH was added. The tube was sealed and kept at room temperature for 15 min. Evaluation of aroma was performed by panelists and scored from grades of 1 to 9.
Data analysis
Analysis of variance (ANOVA) was performed to test the differences in the response to BLB and agronomic traits among the lines and parents. The values of each line were averaged for 10 individuals in each line.
Marker-assisted selection of Xa21 and Xa7
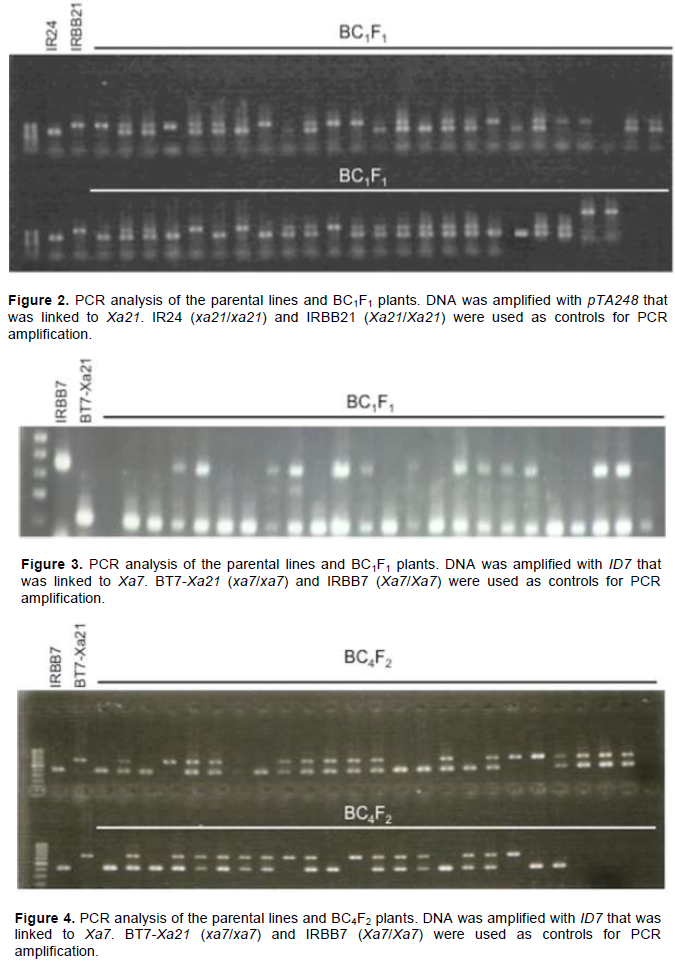
At the BC1F1 generation, a pTA248 marker was used to select plants that were homozygous for Xa21 (Figure 2). In total, 21 out of 96 plants were homozygous for Xa21. These plants were used for marker-assisted selection of Xa7 by using an ID7 marker (Figure 3). Among the 21 plants, 13 were heterozygous at Xa7
An evaluation of agronomic traits were performed for 263 BC2F1 plants and 46 plants that were similar to recurrent parents were selected for genotyping. Among the 46 plants, 21 were heterozygous at Xa7. These plants were backcrossed to recurrent parents to generate a BC3F1 generation. Similarly, among 187 BC3F1 plants, 46 were selected for genotyping. Finally, 13 plants that were heterozygous for Xa7 were backcrossed to recurrent parents to generate a BC4F1 generation. The seeds of the BC4F1 generation were planted to generate 302 BC4F2 lines. Among them, 45 were first selected based on the agronomic traits and were then checked for Xa7 (Figure 4). Finally, 14 lines were sown separately into 14 BC4F3 lines.
Artificial inoculation of BC4F3 lines carrying Xa21 and Xa7
Three Xoo races, which were virulent to IR24 were used for inoculation. Nine lines showed high levels of resistance to race 3 (Table 2). Twelve lines were resistant to races 5 and 14, and two lines were moderately resistant to race14. Recurrent parent BT7-Xa21 was resistant to race 3, moderately resistant to race 5, and susceptible to race 14. Pyramided lines of BT7 carrying Xa21 and Xa7 have acquired novel resistance to race 14 as well as higher resistance to race 3 (e.g., D1, D2, D3, D6, D7, D8, D9, D10 and D12).
Purification and agronomic traits of improved lines at BC4F3 generation
Agronomic traits including plant height, tillers per hill, length and width of flag leaf, effective tillers per hill, growth duration, panicle length, number of fruiting seeds per panicle, seed set rate, 1000-grain weight, and yield were evaluated at the BC4F3 generation (Tables 3 and 4). All lines examined showed good uniformity with purification scores ranging from 5 to 9 even though uniformity of BT7-Xa21 was superior to the improved lines. Plant height was classified into a dwarf group, and the difference to recurrent parent ranged from 3.0 (D13) to 10.7 cm (D5). Tillering ability of the lines was similar to the recurrent parent BT7-Xa21, and the number of tillers per hill varied from 9.5 (D7) to 10.7 tillers (D10), while that of recurrent parent was 10.3 tillers. Effective tillers per hill varied from 7.6 (D12) to 8.7 (D3). The growth duration of improved lines was similar to the recurrent parent, but uniformity was less than the recurrent parent. Based on these agronomic traits, D2, D3, D6, D8, D9 and D13 were selected as promising lines.
Yield and yield components of improved lines were similar to those of recurrent parent BT7-Xa21. Moderate panicle size, high seed set ratios between 81.4 (D14) and 90.8 (D7), small seeds, and number of spikelets per panicle ranging from 124.1 (D13) to 142.7 (D9) were observed. 1000-grain weight varied from 20.1 to 20.8 g. Yield of improved lines varied from 61.1 (quintal/ha) (D13) to 75.8 (quintal/ha) (D10) even though the control was 71.5 (quintal/ha). Finally, D2, D6, D8 and D9 were selected as promising lines for quality evaluation based on response to BLB, phenotypic uniformity, agronomic traits and yield. The D3 and D13 lines were excluded due to some inferior quality traits (data not shown).
Quality evaluation of improved lines at BC4F3 generation
BT7-Xa21 is a high-quality rice variety with slender, small, soft, brownish and aromatic grains, and is almost identical to the original cultivar BT7 except for the possession of Xa21. Some indicators of high-quality varieties are presented in Table 5. The four selected lines had grain characteristics similar to recurrent parent BT7-Xa21 including a brown yellow hull, seed length of 6.2 to 6.3 mm, and length/width of 2.7 to 2.8 (Table 5). Two of the promising lines, D6 and D9, had the same level of aroma as produced by recurrent parent BT7-Xa21. Based on the level of BLB resistance and agronomic traits, two lines, D6 and D9, were eventually selected as promising lines to be released to farmers’ fields.
In this study, plants carrying Xa7 in addition to Xa21 from BC1F1 to BC4F2 generations were successfully selected using the MAS technique. Plants carrying two resistance genes show wider resistance than plants carrying single resistance gene. Previously, pyramiding BLB resistance genes, Xa4 and xa5, or xa5 and Xa10, was shown to express higher levels of resistance to BLB than a single gene (Huang et al., 2012). Similarly, the combination of Xa21 and Xa7 showed a high level of resistance as well as wide spectrum resistance to BLB (Table 2). Furthermore, the improved lines showed high phenotypic uniformity, semi-dwarf, good tillering, high seed set rate and small seeds like the recurrent parent at the BC4F3 generation. This proved that pyramiding two resistance genes Xa7 and Xa21 was useful for improving BLB resistance in cultivar BT7. Conventional breeding is laborious, time consuming and difficult to apply when it comes to pyramiding dominant genes with similar reactions to BLB (Collard and Mackill, 2008). he results of this study show that MAS is an effective method to overcome the limitations of phenotypic selection in BLB resistance breeding in rice. Through further improvement of several traits along with additional field trials, the promising D6 and D9 lines will be released as new high quality varieties with improved resistance to BLB.
The authors declare that there is no conflict of interest.
We would like to thank to the Editage (www.editage.jp) for English language editing of the manuscript.
REFERENCES
|
Collard BCY, Mackill DJ (2008). Marker-assisted selection: an approach for precision plant breeding in the twenty first century. Philosophical Transactions of the Royal Society of London B: Biological Sciences 363:557-572.
Crossref
|
|
|
|
Furuya N, Taura S, Goto T, Thuy BT, Ton PH, Tsuchiya, Yoshimura A (2012). Diversity in virulence of Xanthomonas oryzae pv. oryzae from Northern Vietnam. Japan Agricultural Research Quarterly 46(4):329-338.
Crossref
|
|
|
|
|
Huang B, Xu JY, Hou MS, Ali J, Mou TM (2012). Introgression of bacterial blight resistance genes Xa7, Xa21, Xa22 and Xa23 into hybrid rice restorer lines by molecular marker-assisted selection. Euphytica 187:449-459.
Crossref
|
|
|
|
|
Khan MA, Naeem M, Iqbal M (2014). Breeding approaches for bacterial leaf blight resistance in rice (Oryza sativa L.), current status and future directions. European Journal of Plant Pathology 139:27-37.
Crossref
|
|
|
|
|
Kibria K, Iskam MM, Begum SN (2008). Screening of aromatics rice lines by phenotypic and molecular markers. Bangladesh Journal of Botany 37:141-147.
Crossref
|
|
|
|
|
Lee SW, Choi SH, Han SS, Lee DG, Lee BY (1999). Distribution of Xanthomonas oryzae pv. oryzae strains virulent to Xa21 in Korea. Phytopathology 89:928-933.
Crossref
|
|
|
|
|
Marella LS, George MLC, Vera Cruz CM, Bernardo MA, Nelson RJ, Leung H, Reddy JN, Sridhar R (2001). Identification of resistance genes effective against rice bacterial blight pathogen in Eastern India. Plant Disease 85:506-512.
Crossref
|
|
|
|
|
Mew TW, Vera Cruz CM, Medalla ES (1992). Changes in race frequency of Xanthomonas oryzae pv. oryzae in response to the planting of rice cultivars in Philippines. Disease 76:1029-1032.
|
|
|
|
|
Mew TW, Wu SZ, Horino O (1982). Current status and future prospects of research on bacterial blight of rice. Annual Review of Phytopathology 25:359-382.
Crossref
|
|
|
|
|
Monna L, Kitazawa N, Yoshino R, Suzuki J, Masuda H, Maehara Y, Tanji M, Sato M, Nasu S, Miobe Y (2002). Positional cloning of rice semidwarf gene, sd-1: rice "green revolution gene" encodes a mutant enzyme involved in gibberellin synthesis. DNA Research 9:11-17.
Crossref
|
|
|
|
|
Pradhan SK, Nayak DK, Mohanty S, Behera L, Barik SR, Pandit E, Lenka S, Anandan A (2015). Pyramiding of three bacterial blight resistance genes for broad-spectrum resistance in deepwater rice variety, Jalmagna. Rice 8(1):19.
Crossref
|
|
|
|
|
Sirivastava DN (1972). Steak of rice. Central Rice Research Institute Cuttack, Orrisa, Indica P. 143.
|
|
|
|
|
Varghese YA, Knaak C, Sethuraj MR, Ecke W (1997). Evaluation of random amplified polymorphic DNA (RAPD) markers in Hevea brasiliensis. Plant Breeding 116:47-52.
Crossref
|
|
|
|
|
Vera Cruz CM, Bai J, O-a I, Leung H, Nelson RJ, Mew TW, Leach JE (2000). Predicting durability of a disease resistance gene based on an assessment of the fitness loss and epidemiological consequences of avirulence gene mutation. Proceedings of the National Academy of Sciences of the United States of America 97:13500-13505.
Crossref
|
|
|
|
|
Webb KM, Ona I, Bai J, Garrett KA, Mew T, Vera Cruz CM, Leach JE (2010). A benefit of high temperature: increased effectiveness of a rice bacterial blight disease resistance gene. New Phytologist. 185:568-576.
Crossref
|
|
|
|
|
Williams CE, Wang B, Holsten TE, Scambray de Assis Goes da Silva F, Ronald PC (1992). Markers for selection of the rice Xa21 disease resistance gene. Theoretical and Applied Genetics 93:1119-1122.
Crossref
|
|
|
|
|
Xu J, Jiang J, Dong X, Ali J, Mou T (2012). Introgression of bacterial blight (BB) resistance genes Xa7 and Xa21 into popular restorer line and their hybrids by molecular marker-assisted backcross (MABC) selection scheme. African Journal of Biotechnology 11:8225-8233.
|
|
|
|
|
Yara A, Phi CN, Matsumura M, Yoshimura A, Yasui H (2010). Development of near-isogenic lines for BPH25(t) and BPH26(t), which confer resistance to the brown planthopper, Nilaparvata lugens (Stål) in indica rice 'ADR52'. Breeding Science 60:639-647.
Crossref
|
|
|
|
|
Zhang Y, Wang J, Pan J, Gu Z, Chen X, Jin Y, Liu F, Zhang H, Ma B (2009). Identification and molecular mapping of the rice bacterial blight resistance gene allelic to Xa7 from an elite restorer line Zhenhui 084. European Journal of Plant Pathology 125:235-244.
Crossref
|
|