ABSTRACT
The effect of pasteurization-range heat treatment on yeast inactivation, vitamin C and the physicochemical characteristics of fresh pineapple juice were assessed. Yeast inactivation could be described by the Weibull model. The desired 6 log reduction was achieved at 63 and 65°C for 8 and 2 min, respectively. The pH, degree brix and organic acids did not change from 55 to 95°C. A significant change in fructose and glucose contents started to occur at 85°C, while sucrose hydrolysis was observed from 95°C. Likewise, hydroxymethylfurfural, one of the intermediate products of the Maillard reaction, was detected at 95°C. Little degradation of ascorbic acid, the most important nutrient in pineapple juice- was observed. Hence, a mild heat treatment of 2 min at 65°C was sufficient to inactivate yeast and to preserve the nutritional and physicochemical quality of the pineapple juice.
Key words: Pineapple juice, heat treatment, yeast inactivation, physicochemical and nutritional quality.
Pineapple is one of the most appreciated tropical fruit due to its exotic aroma and flavour (Rattanathanalerk et al., 2005). The fruit can be consumed fresh or processed into many products including canned slices, juice concentrate, pulp, dried parts and pasteurized juice. The latter is the most popular due to its pleasant sensorial attributes.
Heat treatments are generally applied to extend the shelf life of fruit products. Regarding pineapple juice, they mostly target yeast because lactic acid bacteria are less heat resistant (Aneja et al., 2014; Hounhouigan et al., 2014a). However, heat treatment can affect nutritional and sensory quality attribute: vitamin C losses and nonenzymatic browning are reported as the main consequences of thermal treatment on pineapple juice (Rattanathanalerk et al., 2005; Wurlitzer et al., 2019). Nowadays, new technologies are being developed to avoid nutritional and sensorial losses, such as ultrasound, high pressure, ultraviolet radiation, pulsed light and pulsed electric fields (Gopal et al., 2017; Zhang et al., 2018; Indriani et al., 2018). However, thermal pasteurization remains important because it is a simple and inexpensive technique for small agro-industries. Therefore, it is relevant to assess how pineapple juice can be processed through pasteurization to avoid spoilage while preserving its nutritional and physicochemical quality.
The effect of heat treatment on hydroxymethylfurfural (HMF) and brown pigment accumulation in pineapple juice was investigated in the literature (Rattanathanalerk et al., 2005). However, the authors used a spectrophotometric method, which is not very specific and could have been disturbed by other compounds (Zappala et al., 2005). As for the sensory properties of pineapple juice, organic acids such as citric and malic acids are known to provide the basic acid taste that characterizes the juice (De Vasconcelos Facundo et al., 2010). High concentrations of organic acids and low pH in most fruits are known to be critical for the preservation of fruit juice (Igual et al., 2010). They also help to stabilize ascorbic acid and anthocyanins (Wang et al., 2007).
Kinetic studies are helpful to predict quality loss resulting from different process conditions. For instance, the application of the Weibull model to describe thermal inactivation of microbial vegetative cells has been investigated (Dementavicius et al., 2016) and kinetic models have been used to evaluate vitamin C degradation in fruit products such as orange juice (Vikram et al., 2005), strawberry juice (Odriozola-Serrano et al., 2008) and citrus juice (Burdurlu et al., 2006). Despite the multitude of research on fruit juice preservation (Aleman et al., 1996; Linton et al., 1999; Nguyen et al., 2019), information regarding yeast inactivation, kinetics of vitamin C degradation, the change of sugars, organic acids and HMF using a more specific method such as High Performance Liquid Chromatography (HPLC) are lacking for pasteurized pineapple juice. The aim of this work is to investigate the effect of heat treatment in the pasteurization range on yeast inactivation and the physicochemical and nutritional quality-mainly ascorbic acid- of pineapple juice.
Yeast inactivation
Raw material
Three batches of fresh and mature pineapples, variety ‘Kona Sugarloaf’, from different production areas in Benin, West Africa were used to perform this experiment. The pineapples were processed immediately after purchase.
Juice preparation
After rinsing the fruits in tap water, the shell was removed using a stainless steel knife. The flesh was cut into small pieces and the juice was extracted using a hydraulic machine (Compact Health Stream Juice press, UK) at ambient room temperature (28°C).
Heat treatment
A series of glass tubes was filled with 15 mL of fresh pineapple juice and subjected to heat treatments in a heating block (Liebisch Labortechnik, the Netherlands) at 57, 59, 61, 63 and 65°C, respectively for different time periods. The juice was treated at 57°C for 5, 10, 15, 20 and 25 min; at 59°C for 1, 2, 4, 6, 8 and 10 min; at 61°C for 1, 2, 3, 4 and 5 min; at 63°C for 25, 40, 60 80, and 100 s; and finally at 65°C for 10, 20 and 30 s. After the treatment time the tubes were immediately cooled in an ice-water bath.
Yeast counts
Ten millilitres of fresh pineapple juice was transferred aseptically into 90 mL sterile peptone salt (Merck Millipore) (5 g of peptone, 8.5 g of NaCl, 1000 mL of distilled water, pH = 7.2 ± 0.2) and homogenized for about one minute using a stomacher (Stomacher 400 circulator Seward, England). Yeasts were enumerated by the pour plate method. Yeasts and moulds were grown on Malt Extract Agar (MEA) (Merck Millipore) incubated at 25°C for 72 h (Heard and Fleet, 1985; Skaar and Stenwig, 1996).
Chemical changes in pasteurized juice
Juice preparation
Three batches of ‘Kona Sugarloaf’, purchased in Benin from three different farmers to perform this experiment in triplicate were stored at a temperature between 12 and 16°C. Batch (A) was processed into juice on the first day after the arrival in the Netherlands (day 1). On day 2, batch (B) was processed and on day 3, batch (C). After juice preparation, the samples were stored frozen at –20 °C, except for the samples in which vitamin C was determined.
Heat treatment
Triplicate experiments were conducted at five temperatures (55, 65, 75, 85 and 95°C) and seven time periods (0, 10, 20, 30, 40, 50 and 60 min), using the three batches of pineapple (A, B and C). A 5 x 7 factorial design was used in the scheduling of each experiment. For each experiment, five series of seven glass tubes were filled with 8 mL of fresh pineapple juice, sealed with tube caps and subjected to heat treatment in a heating block (Liebisch Labortechnik, the Netherlands) at 55, 65, 75, 85 and 95°C, respectively, from 10 to 60 min. After treatment, the tubes were immediately cooled in an ice-water bath. Vitamin C, pH, degrees brix, malic acid, citric acid, HMF, fructose, glucose and sucrose were measured.
pH, degree brix, citric and malic acids determination
The pH was measured using a pH meter (Inolab PH 720) at room temperature. The degree brix was measured with a refractometer (Bellingham-Stanley Eclipse equipment). Organic acids (citric and malic acids) were determined by HPLC. The prepared pineapple juice samples were first centrifuged for 10 min at 1000 rpm and then supernatants were filtered with a 0.2 µm cellulose acetate (CA) filter. The solution was poured in a vial and analysed by HPLC equipped with a Prevail Organic Acids Column 250 × 4.6 mm Grace Alltech 88645 and a Dionex Ultimate 3000 RS Diode-Array Detector(DAD) monitored at 210 nm. The analytical conditions were : flow 1 ml min-1, isocratic mobile phase, a column temperature of 30°C, eluent 0.1 M KH2PO4 in Milli-Q water of which the pH was adjusted to pH 2.5 using phosphoric acid.
Vitamin C determination
Vitamin C consists of ascorbic acid (AA) and its oxidized form, dehydroascorbic acid (DHA). Fresh pineapple juice (1 mL) was collected into a 1.5 ml Greiner tube and then centrifuged for 10 min at 10,500 rpm and 4°C. The supernatant was collected and filtered with a 0.2 µm CA filter; 0.5 ml of the filtered supernatant was transferred into a glass tube that contained 7.5 ml of 3% metaphosphoric acid (MPA) (Merck Millipore) and 1 mM of tert-butylhydroquinone (Merck Millipore) (THBQ). The vitamin C concentration was determined by measuring ascorbic acid (AA) using an HPLC system equipped with a C18a Polaris Column 150 × 4.6 mm and an UV detector monitored at 245 nm. The analytical conditions were flow 1 ml min-1, isocratic mobile phase, eluent 0.2% (v/v) orthophosphoric acid in Milli-Q water, and a column temperature of 20°C. Then, DHA was reduced back to AA; for that purpose, 1.5 ml of filtered fruit juice was mixed with 15 µl 1M Tris-2-carboxyethylphosphine (TCEP) (Merck Millipore) solution in amber HPLC vials and stored in the dark for at least 20 min in ice (Hounhouigan et al., 2014b)
Sugar determination
Pineapple juice was diluted 400 times with Milli-Q water. These solutions were filtered using a 0.2 µm CA filter. The filtrate’s contents of glucose, fructose and sucrose were determined by HPLC using an Alltech Prevail Carbohydrates ES 5 µm 250 × 4.6 mm column equipped with an Evaporative Light Scattering Detector (ELSD). The temperature of the evaporator was set at 80°C, the temperature of the nebulizer at 60°C, and the carrier flow rate was 1.3 ml min-1. The analytical conditions were: flow 1 ml min-1, isocratic mobile phase, a column temperature of 25°C, and an eluent 75% (v/v) acetonitrile in demineralized water.
Hydroxymethylfurfural determination
The juice samples were centrifuged for 10 min at 1000 rpm; the supernatant was filtered with a 0.2 µm CA filter and transferred into HPLC vials. The HMF contents of the filtrates were determined by HPLC equipped with a Varian C18a Polaris Column 150 × 4.6 mm and UV/VIS detector monitored at 284 nm. The analytical conditions were: flow 1 ml min-1, isocratic mobile phase, eluent 5% (v/v) acetonitrile in Milli-Q water, and a column temperature of 20°C.
Kinetic modelling of yeast inactivation
The kinetics of yeast inactivation in the pineapple juice during heat treatment was described according to the Weibull model (Van Boekel, 2009). The survival curves were obtained by plotting the log (N/No) (with N the number of survivors and No, the initial number) versus time (min) for each temperature. The parameters in the Weibull model were obtained via nonlinear least-square regression using the solver option in Excel (Microsoft). The error analysis on the parameters was done using the Excel macro solverAid (De Levie, 2004). The cumulative function of the survival curve used is:
in which d is the number of decimal reductions.
Statistical analysis
Results are given as mean ± SD of three independent determinations. A one-way Anova was used to test the effect of heat treatment on the different physicochemical and nutritional parameters of the pasteurized pineapple juice.
Effect of heat treatment on the inactivation of yeasts
The Weibull model has been proposed to describe non-linear inactivation curves (Dementavicius et al., 2016; Li et al., 2018)The model is sufficiently robust to describe a concave upward survival curve if β < 1 and a concave downward if β > 1 (Dementavicius et al., 2016). Figure 1 shows the data and the fit of the Weibull model to yeast inactivation at 57, 59, 61, 63 and 65°C. Two curves resulted in β < 1 and three in β > 1. At 57 and 59°C, at which β < 1 (Figure 1a to b); the results suggest that the remaining cells of the yeast seem to resist the heat stress. However, at the higher temperatures 61, 63 and 65°C, at which β > 1, the opposite is the case (Figure 1c to e); the remaining cells seem to become increasingly damaged at increasing heating time. Visual inspection of the curves indicates that the fits obtained are very reasonable; the residuals are, by and large, randomly distributed. It is also obvious that first order kinetics does not apply to the data in Figure 1a to e. The calculated Weibull model parameters with corresponding correlation coefficients between the parameters are listed in Table 1.
The results show a transition in heat resistance at 61°C with a shift from b < 1 to b > 1. This is an interesting phenomenon because it shows that something critical happens to the cells at temperatures above 61°C. Unfortunately, it makes the temperature dependence of the Weibull parameters complicated. The parameters a and b are correlated: if b changes, a does as well. If the parameter b remains constant, the temperature dependence of a can be modelled in a straightforward way: there is a logarithmic relation between a and temperature (Van Boekel, 2002; Oliveira et al., 2018). However, because of the correlation between a and b and the fact that b is not constant, this relation is disturbed, as shown in Figure 2.
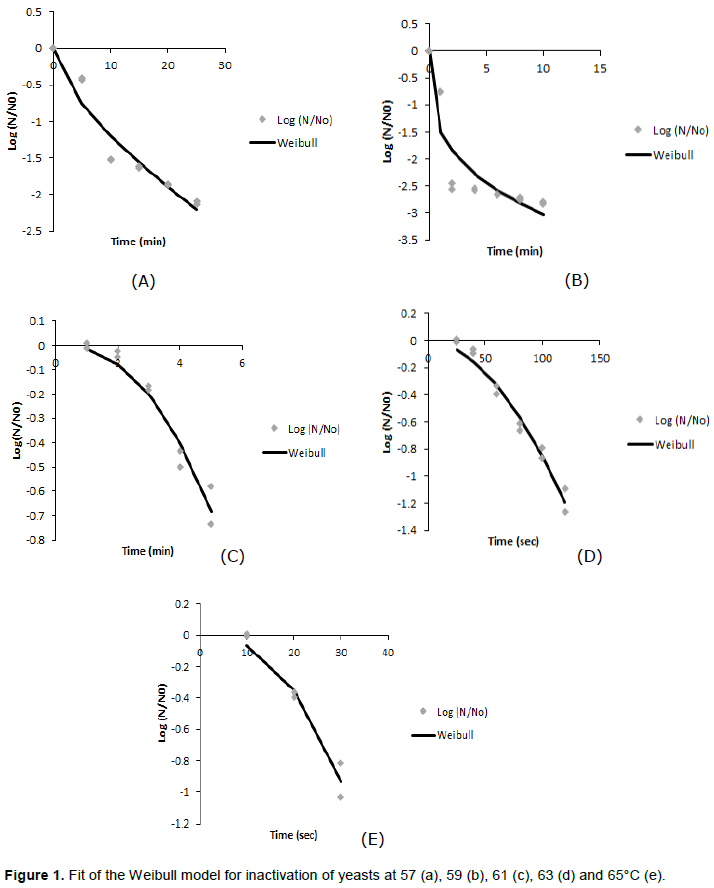
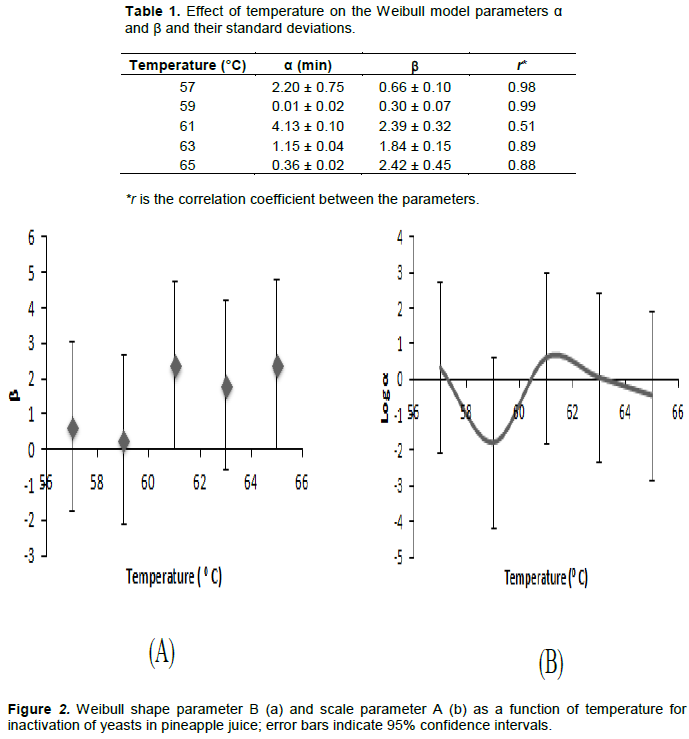
The time needed to achieve a 6 log reduction of yeast in the heated pineapple juice was 37 min at 57°C, 8 min at 63°C and 2 min at 65°C. D-values from literature are D60 °C < 1 min for yeast in general and D60°C = 7-22 min for the strain Saccharomyces cerevisiae (Hocking and Jensen, 2001). Despite the complications in kinetics, the results suggest that a heat treatment of 65°C for 2 min is sufficient to inactivate yeast in pineapple juice and therefore to give juice a proper microbial shelf life by pasteurization. The remaining question is what happens to the non-microbial characteristics of the juice. This is discussed in the following sections.
Effect of heat treatment on physicochemical characteristics
PH, degree Brix, citric and malic acids
The initial pH of the juice was 3.86 ± 0.05 and the degree brix was 11.0 ± 1.5. The pH and the degree brix did not change significantly (P > 0.05) due to the heat treatments of the pineapple juice, which is in line with other studies (Yeom et al., 2000).These quality parameters are important as they are closely related to the stability of bioactive compounds in fruit products (Kaddumukasa et al., 2017). There were no changes in the malic and citric acids contents after the heat treatments; on average these were 0.02 ± 0.002 M and 0.031 ± 0.005 M, respectively.
Vitamin C
Figure 3 shows the change in the AA concentration during heat treatments at 55, 65, 75, 85 and 95°C in pineapple juice. AA significantly (P < 0.05) decreased from 0.91 ± 0.32 mM at 55°C to 0.73 ± 0.26 mM at 95°C. In addition, a significant difference (P < 0.05) was observed in the AA content when juice was heated at 65, 75 and 85°C (Figure 3). This result suggests that the increase in temperature affects the AA in pineapple juice to some extent. AA is thermolabile and several studies have reported the effect of temperature on AA content (Vikram et al., 2005). Even though the total loss of vitamin C between the highest and the lowest temperatures studied was around 20%, the losses in AA from a temperature to another were very low (< 8%). The low degradation rates made it impossible to do a proper kinetic modelling; generally, degradation must be at least 30-40% before a model can be applied (Van Boekel, 2008). A very rough calculation of activation energy from our data shows this to be < 20 kJ.mol-1. However, this only indicates that the temperature sensitivity of AA degradation in pineapple juice is very low; because of the low degradation rates, a precise calculation of the activation energy is not possible. This is a remarkable result because AA is usually susceptible to thermal degradation. Probably, the low pH conditions, as is the case in pineapple juice (3.86 ± 0.05), might stabilize it. A similar behaviour of ascorbic acid was reported in orange juice (Plaza et al., 2006)and marula juice (Hiwilepo-Van Hal et al., 2012). It is also reported that the degradation of vitamin C is very low in citrus juice, in contrast to their expectation (Dhuique-Mayer et al., 2007). Perhaps it is time to reconsider the general perception that vitamin C is always very thermolabile.
The AA content of the pineapple juice treated at 55°C was initially 0.82 mM in batch A, 1.02 mM in batch B and 0.99 mM in batch C. The standard deviation appeared to be very large (± 0.10 mM), but this is not due to the treatment or the analysis method but to the natural variation in content. This high variation in the initial vitamin C was also found in the juice treated at 65, 75, 85 and 95°C. This indicates that the variation between the three batches was inherent in the pineapple juice.
Hydroxymethylfurfural (HMF)
As non-enzymatic browning (Maillard reaction) is one of the major causes of colour change in fruit products, the effect of heating time on the accumulation of HMF was investigated. It was observed that HMF formation was initiated only at 95°C with a minimum time of 30 min. HMF generation can only occur at temperatures above 95°C in orange juice (Marcotte et al., 1998). However, this result deviates from the findings in the literature (Rattanathanalerk et al., 2005)that detected, by spectrophotometry, HMF in pineapple juice treated at 55°C. This could be due to the HMF detection method. Spectrophotometry may suffer from interferences from other compounds that absorb light at the same wavelength as HMF. Figure 4 shows the effect of heating at 95°C on the HMF content (with standard deviation for 3 replicates) in pineapple juice.
Fructose, glucose and sucrose
Sucrose, glucose and fructose were studied as they are the major sugar components of pineapple juice (Khalid et al., 2016). The concentrations of fructose (123 ± 5.1 mM), glucose (100.5 ± 4.7 mM) and sucrose (320.8 ± 8.1 mM) in pineapple juice did not change after treatments at 55, 65 and 75°C (Figure 5). At 85°C, sucrose showed a significant (P < 0.05) decrease from 320 to 292.4 mM. Overall, the glucose and fructose significantly increased at 95°C (20, 30, 40, 50 and 60 min) (Figure 5), whereas the sucrose content significantly decreased at 85°C (30, 40 and 50 min) and 95°C (20, 30, 40, 50 and 60 min) (Figure 5). The decrease of sucrose with a simultaneous increase of fructose and glucose is explained by the hydrolysis of sucrose. Obviously, reducing sugars, glucose and fructose are also subject to degradation.
However, our results show that this is very limited at the heat treatments applied here, in line with the fact that HMF formation is very limited. Since fructose is the sweetest of all naturally occurring carbohydrates (Hanover and White, 1993), pasteurization of pineapple juice starting from 85°C might affect the taste of the fresh pineapple juice, making it sweeter than its initial taste. Figure 5 shows the changes in sugars in pineapple juice during heat treatment (with the standard deviation for three replicates).
A heat treatment of 65°C for 2 min appeared to be sufficient to achieve a 6 log reduction of yeast in pineapple juice. Chemical characteristics such as pH, degree brix, malic and citric acids concentrations were not affected by the heat treatments in the range studied. Hydrolysis of sucrose and formation of HMF was observed at 85 and 95°C respectively, which could indicate the start of the Maillard reaction. The vitamin C content decreased with heating temperature but the effect of temperature on vitamin C degradation was low in the temperature range studied. Therefore, a pasteurization treatment at a relatively low temperature could be used to prevent pineapple juice from yeast spoilage while preserving its nutritional and physicochemical quality. These results are of importance for pineapple juice processors in small and large-scale juice industries. Most likely, pineapple juice is over-processed. Our results show that pasteurization does not need to be severe in order to obtain a reasonable shelf life. We did not study the organoleptic quality of the juice, but since pasteurization can be done at a rather low temperature, we anticipate that damage to organoleptic quality will be limited.
The authors have not declared any conflict of interests.
REFERENCES
|
Aleman GD, Ting EY, Mordre SC, Hawes ACO, Walker M, Farkas DF, Torres JA (1996). Pulsed ultra-high pressure treatments for pasteurization of pineapple juice. Journal of Food Science 61:388-390.
Crossref
|
|
|
|
Aneja KR, Dhiman R, Aggarwal NK, Aneja A (2014). Emerging Preservation Techniques for Controlling Spoilage and Pathogenic Microorganisms in Fruit Juices. International Journal of Microbiology 14.
Crossref
|
|
|
|
|
Burdurlu HS, Koca N, Karadeniz F (2006). Degradation of vitamin C in citrus juice concentrates during storage. Journal of Food Engineering 74:211-216.
Crossref
|
|
|
|
|
De Levie R (2004). Advanced Excel for Scientific Data Analysis. Oxford University Press, New York.
|
|
|
|
|
De Vasconcelos Facundo HV, De Souza Neto MA, Maia Narendra Narain GA, Dos Santos Garruti D (2010). Changes in flavor quality of pineapple juice during processing. Journal of Food Processing and Preservation 34:508-519.
Crossref
|
|
|
|
|
Dementavicius D, Lukseviciute V, Gomez-Lopez VM, Luksiene Z (2016). Application of mathematical models for bacterial inactivation curves using Hypericin-based photosensitization. Journal of Applied Microbiology 120:1492-1500.
Crossref
|
|
|
|
|
Dhuique-Mayer C, Tbatou M, Carail M, Caris-Veyrat C, Dornier M, Amiot MJ (2007). Thermal degradation of antioxidant micronutrients in citrus juice: kinetics and newly formed compounds. Journal of Agricultural and Food Chemistry 55:4209-4216.
Crossref
|
|
|
|
|
Gopal KR, Kalla AM, Srikanth K (2017). High Pressure Processing of Fruits and Vegetable Products: A Review. International Journal of Pure and Applied. Bioscience 5(5):680-692.
Crossref
|
|
|
|
|
Hanover LM, White JS (1993). Manufacturing, composition, and applications of fructose. The American Journal of Clinical Nutrition 58:724S-732S.
Crossref
|
|
|
|
|
Hiwilepo-Van Hal P, Bosschaart C, Van Twisk C, Verkerk R, Dekker M (2012). Kinetics of thermal degradation of vitamin C in marula fruit (Sclerocarya birrea subsp. caffra) as compared to other selected tropical fruits. LWT - Food Science and Technology 49:188-191.
Crossref
|
|
|
|
|
Hocking AD, Jensen N (2001). Spoilage of various food classes : soft drinks, cordials, juices, bottled water and related products. Spoilage of Processed Food : Causes and Diagnosis. CJ Moir and DC Waterloo: Australian Institute of Food Science and Technology Incorporated Food Microbiology Group AIFST Inc. pp. 187-198.
|
|
|
|
|
Hounhouigan MH, Linnemann AR, Soumanou MM, Van Boekel MAJS (2014a). Effect of processing on the quality of pineapple juice. Food Reviews International 30:112-133.
Crossref
|
|
|
|
|
Hounhouigan MH, Linnemann AR, Ingenbleek PT, Soumanou MM, Van Trijp HC, Van Boekel MA (2014b). Effect of physical damage and storage of pineapple fruits on their suitability for juice production. Journal of Food Quality 37(4):268-273.
Crossref
|
|
|
|
|
Igual M, García-Martínez E, Camacho M, Martínez-Navarrete N (2010). Effect of thermal treatment and storage on the stability of organic acids and the functional value of grapefruit juice. Food Chemistry 118:291-299.
Crossref
|
|
|
|
|
Indriani DW, Amalia S, Sumarlan SH, Barunawati N (2018). Effect of Voltage and Frequency in Pasteurization Pulsed Electric Field (PEF) Continous System of Pineapple (Ananas comosus [L.] Merr) Juice. IOP Conference Series: Materials Science and Engineering.
Crossref
|
|
|
|
|
Kaddumukasa PP, Imathiu SM, Mathara JM, Nakavuma JL (2017). Influence of physicochemical parameters on storage stability: Microbiological quality of fresh unpasteurized fruit juices. Food Science and Nutrition 5:1098-1105.
Crossref
|
|
|
|
|
Khalid N, Suleria HAR, Ahmed I (2016). Pineapple Juice. Handbook of Functional Beverages and Human Health. pp. 489-500.
Crossref
|
|
|
|
|
Li R, Kou X, Zhang L, Wang S (2018). Inactivation kinetics of food-borne pathogens subjected to thermal treatments: a review. International Journal of Hyperthermia 34(2):177-188.
Crossref
|
|
|
|
|
Linton M, McClements J, Patterson M (1999). Inactivation of Escherichia coli O157: H7 in orange juice using a combination of high pressure and mild heat. Journal of Food Protection 62:277-279.
Crossref
|
|
|
|
|
Marcotte M, Stewart B, Fustier P (1998). Abused Thermal Treatment Impact on Degradation Products of Chilled Pasteurized Orange Juice. Journal of Agricultural and Food Chemistry 46:1991-1996.
Crossref
|
|
|
|
|
Nguyen BT, Bujna E, Fekete N, Tran ATM, Rezessy-Szabo JM, Prasad R, Nguyen QD (2019). Probiotic Beverage From Pineapple Juice Fermented With Lactobacillus and Bifidobacterium Strains. Frontiers in Nutrition.
Crossref
|
|
|
|
|
Odriozola-Serrano I, Soliva-Fortuny R, Gimeno-AñoÌ V, MartiÌn-Belloso O (2008). Kinetic study of anthocyanins, vitamin C, and antioxidant capacity in strawberry juices treated by high-intensity pulsed electric fields. Journal of Agricultural and Food Chemistry 56:8387-8393.
Crossref
|
|
|
|
|
Oliveira RBA, Baptista RC, Chincha AAIA, Conceiç~ao DA, Nascimento JS, Costa LEO, Cruz AG, Sant'Ana AS (2018). Thermal inactivation kinetics of Paenibacillus sanguinis 2301083PRC and Clostridium sporogenes JCM1416MGA in full and low fat "requeij~ao cremoso". Food Control 84:395-402.
Crossref
|
|
|
|
|
Plaza L, Sánchez-Moreno C, Elez-Martínez P, De Ancos B, Martín-Belloso O, Cano MP (2006). Effect of refrigerated storage on vitamin C and antioxidant activity of orange juice processed by high-pressure or pulsed electric fields with regard to low pasteurization. European Food Research and Technology 223:487-493.
Crossref
|
|
|
|
|
Rattanathanalerk M, Chiewchan N, Srichumpoung W (2005). Effect of thermal processing on the quality loss of pineapple juice. Journal of Food Engineering 66:259-265.
Crossref
|
|
|
|
|
Van Boekel MA (2009). Kinetic modeling of reactions in foods. CRC PressI Llc.
Crossref
|
|
|
|
|
Van Boekel MAJS (2008). Kinetic modeling of food quality: a critical review. Comprehensive Reviews in Food Science and Food Safety 7:144-158.
Crossref
|
|
|
|
|
Vikram VB, Ramesh MN, Prapulla SG (2005). Thermal degradation kinetics of nutrients in orange juice heated by electromagnetic and conventional methods. Journal of Food Engineering 69:31-40.
Crossref
|
|
|
|
|
Wang YC, Chuang YC, Ku YH (2007). Quantitation of bioactive compounds in citrus fruits cultivated in Taiwan. Food Chemistry 102:1163-1171.
Crossref
|
|
|
|
|
Wurlitzer NJ, Dionı'sio AP, Lima JR, dos Santos Garruti D, da Silva Arau'jo IM, Julia˜o da Rocha RF, Lima Maia J (2019).Tropical fruit juice: effect of thermal treatment and storage time on sensory and functional properties. Association of Food Scientists and Technologists.
Crossref
|
|
|
|
|
Yeom HW, Streaker CB, Zhang QH, Min DB (2000). Effects of pulsed electric fields on the quality of orange juice and comparison with heat pasteurization. Journal of Agricultural and Food Chemistry 48:4597-4605.
Crossref
|
|
|
|
|
Zappala M, Fallico B, Arena E, Verzera A (2005). Methods for the determination of HMF in honey: a comparison. Food Control 16:273-277.
Crossref
|
|
|
|
|
Zhang ZH, Wang LH, Zeng XA, Han Z, Brennan CS (2018). Non-thermal technologies and its current and future application in the food industry: a review. International Journal of Food Science and Technology 54:1-13.
Crossref
|
|