ABSTRACT
Conventional propagation methods of sweet potato (Ipomoea batatas L. var Kulfo) through stem cutting require large amount of propagules and large space for preparation. It has high risk of disease transmission to the next generation. In vitro propagation is the best alternative to overcome such limitations. This study was conducted to optimize protocol for in vitro propagation of Kulfo sweet potato variety using 6-benzylaminopurine (BAP) and indole-3-butyric acid (IBA) phytohormones for shoot multiplication and rooting, respectively. The result revealed that the highest shoot initiation (77.78%) and shoot length (4.40 cm) was observed in MS media supplemented with 0.5 mg/l BAP. Best shoot multiplication (5.33 shoots per explants) was obtained in MS medium supplemented with 1 mg/l BAP. MS medium supplemented with 0.5 mg/l IBA showed 100% rooting and average root length of 7.44 cm in vitro. In ex vitro conditions, 93.33% rooting was recorded. During acclimatization, 84 and 93% survival of in vitro and ex vitro rooted plantlets, respectively, were recorded. It could be concluded that MS media without BAP provides optimal condition for shoot initiation. MS supplemented with 1 mg/l BAP provides optimum condition for shoot multiplication. The ex vitro rooting could be better option to reduce in vitro rooting cost and for increased plantlet survival during acclimatization. Based on the result, 0.5 mg/l BAP for shoot initiation, 1 mg/l BAP of shoot multiplication and directly transferring to soil for rooting was recommended for micropropagation of Kulfo sweet potato variety. However, further studies will be needed in ex vitro root induction.
Key words: Sweet potato, micropropagation, shoot tip culture.
In developing countries, sweet potato is an important part of food security packages (Dagne et al., 2014). It is mainly cultivated for its expanded edible roots which contain high carbohydrate, minerals and vitamins like vitamin A (beta carotene) and C to a large sector of the global population (Tumwegamire et al., 2011; Shonga et al., 2013).
Sweet potato is traditionally multiplied mainly by stem cuttings which are a slow process, and diseases may accumulate in the vine cuttings from generation to generation. This could result in declining of root yield and loss of superior genotypes. This method requires large area, incurs high cost and consumes time. Despite many efforts, the underlying problem in this procedure is low frequency of regeneration, long periods of culture and frequent media changes (Gosukonda et al., 1995).
Micropropagation of sweet potato offers significant advantages in the production of large number of disease free clonal propagules within a short time, with the possibility of eliminating viral, bacterial and fungal infection and the production of high quality and uniform plantlets (Neja, 2009; Tekalign et al., 2012). Kulfo variety is high yielding, orange fleshed sweet potato with high quality in terms of vitamins A and C (Laban et al., 2015). There is high demand of orange fleshed sweet potato in Ethiopia but shortage of planting material is a critical problem. Therefore, the aim of this study was to optimize protocol for rapid micropropagation of Kulfo sweet potato variety in in vitro condition using shoot tip culture.
Plant material
The study was conducted using orange fleshed sweet potato variety, Kulfo. This variety was obtained from Hawassa Agricultural Research Centre, Southern Nations Nationalities and Peoples’ Region, Ethiopia. Vine cuttings of about 25 cm long were planted and grown in greenhouse at the College of Agriculture and Veterinary Medicine, Jimma University, Jimma, Ethiopia. The mother plants were irrigated twice per day and allowed to grow for a month after which actively growing shoot tips were collected and used as source of explants.
Stock solution and medium preparation
MS media (Murashige and Skoog, 1962) supplemented with various plant growth regulators were used. Stock solutions of the macronutrients, micronutrients, vitamins, iron source and plant growth regulators (1 mg: 1 ml) were prepared and stored at +4°C in refrigerator for immediate use. For dissolving the plant growth regulators, drop of ethanol was used for IBA and drop of NaOH for cytokinins (BAP) before making up the final volume with distilled water. The dissolved solution was poured into labeled volumetric flask to be fully dissolved and finally stored in refrigerator for later use.
The culture medium containing 30 g/l sucrose was prepared from their respective stock solutions. The plant growth regulators (BAP and IBA) were added to the medium as required with various concentrations. The mixture was stirred using magnetic stirrer and the volume was adjusted using double distilled water. Then, pH was adjusted in all cases to 5.8 using 1 M NaOH or 1 M HCl. Finally, 8.0 g/l agar was added and heated to melt throughout the experiment. Before autoclaving, the media were dispensed into sterilized culture jars. The media were steam sterilized using autoclave at a temperature of 121°C with a pressure of 0.15 Kpa for 15 min and transferred to the culture room and stored under aseptic conditions for later use.
Sterilization and initiation of the cultures
Healthy vines shoot tip of Kulfo sweet potato variety was collected as explants. The explants were then washed with distilled water and sterilized by dipping in 70% ethanol for 1min in a sterilized jar and washed using sterile distilled water three times for 5 min. They were then sterilized with 1% (v/v) commercial bleach (NaOCl) solution containing 3–4 drops of Tween-20 for 15 min and rinsed 4 times with sterile double distilled water each for 5 min with gentle shaking to remove the chemical residue. The damaged parts were excised off using a sterile scalpel and about 1 cm long explants were cultured into the nutrient media. The cultures were maintained at room temperature in the growth room with white florescent lamps of 16/8h light and dark photoperiod, respectively.
Effect of different concentration of BAP on shoot initiation
The sterilized explants were cultured on basal MS medium supplemented with various concentrations of BAP (0, 0.5, 1.0, 1.5, and 2.0 mg/l). The experiment was laid down in completely randomized design in factorial with three replications with five shoot tips per jar. After 3 weeks, percent shoot initiation and shoot length were recorded.
Effect of different concentrations of BAP on shoot multiplication
For shoot multiplication experiment, the initiated shoots were taken and cultured on hormone free MS medium for two weeks to avoid carry over effects in the next experinment. MS medium supplemented with different concentrations of BAP (0.0, 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 mg/l) were used for shoot multiplication. The experiment was arranged in completely randomized design with three replications with five shoots per jar. The cultures were placed in white florescent light room adjusted at 16/8 h light/dark at room temperature. Shoot number, shoot length and leave number were recorded after 5 weeks of culturing.
Effect of IBA for root formation
For in vitro rooting experiment, well developed shoots were cultured on hormone free MS medium for avoiding carry over effect. The shoots were transferred to half strength MS medium supplemented with 0.0, 0.1, 0.5, 0.75 and 1.0 mg/l IBA. The experiment was laid down as completely randomized design (CRD) with three replications with five shoots per jar were used. For ex vitro rooting experiment, 15 shoots were directly transferred to greenhouse from shoot multiplication media by carefully excising in vitro multiplied micro-shoots for rooting and hardening, simultaneously. After a month, number of roots, root length and percentage of rooted plantlets were recorded for both in vitro and ex vitro treatments.
Acclimatization
Plantlets with well-developed root and leaves were washed with tap water to remove adhering media and sucrose attached on the roots of plantlets. Fifteen plantlets were transferred to plastic pots in greenhouse containing hardening medium composed of soil, compost and sand (1:1:2) ratio, respectively. The plants were placed in pots covered with transparent plastic bags and irrigated using sprayer every day. Plastic cover were removed partially after a week and completely removed after two weeks. Finally, after 30 days, the survival rates of the plantlets were evaluated by counting the number of successfully acclimatized plantlets.
Data analysis
SAS software v9.2 (SAS Institute, 2008) was used for data analysis of variance and significance difference between treatments. Means separation was done with least significance difference (LSD) at 0.01 probability level.
Shoot induction
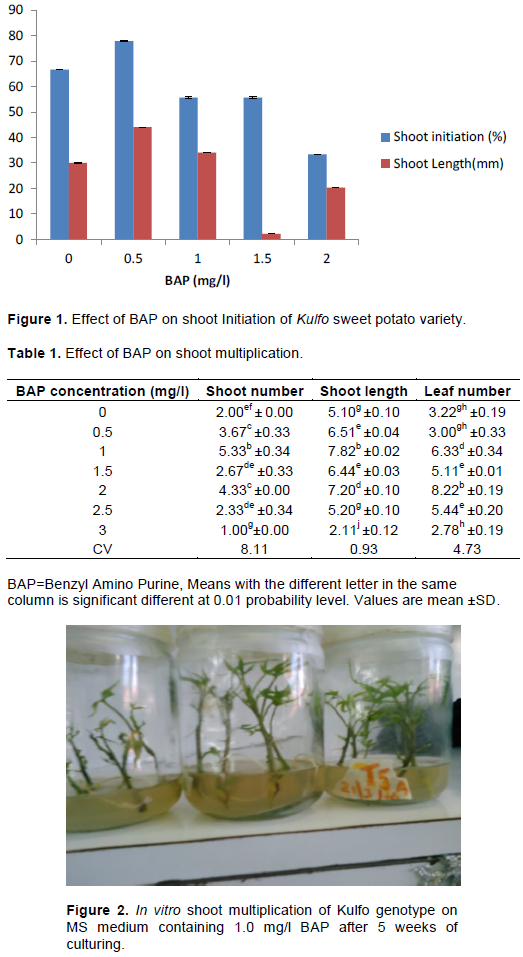
MS medium supplemented with 0.5 mg/l BAP showed the maximum initiated shoots (77.78±0.23) and shoot length (4.40±0.11 cm) per explants (Figure 1).
A decrease in percent shoot initiation was observed for increasing BAP concentrations from 0.5 to 2 mg/l. Low concentrations of BAP were effective in rapid shoot initiation due to activation of cellular process. High concentrations (2 mg/l) of BAP resulted to significantly lower shoot initiation due to the inhibitory effect on metabolism and shoot elongation. This is due to high concentration of BAP that leads to metabolic inhibition. Gosukonda et al. (1995) found that different sweet potato varieties respond differently to in vitro shoot initiation media. However, in many cases higher concentrations of BAP were found to inhibit shoot elongation (George et al., 2008). The result was also in conformity with the finding of Sowal et al. (2002) who reported the effectiveness of low concentration of BAP to result in rapid shoot initiation due to the activation of tRNA cytokinins resulting in rapid proliferation of shoot primordial. In a similar study, Khadiga et al. (2009) also reported that BAP at the concentration of 5 mg/l gives low number of regenerated shoots of sweet potato.
Effects of BAP on shoot multiplication
Maximum shoots per explant (5.33 ±0.34) and shoot length (7.82 ±0.02 cm) were observed on MS media supplemented with 1.0 mg/l BAP (Table 1 and Figure 2). Minimum shoots number (1.00 ±0.00), shoot length (2.11±0.12 cm) and leaves number (2.78 ±0.19) were observed on MS media supplemented with the highest concentration of BAP (3.0 mg/l). At concentration of BAP below 1 mg/l, mean number of shoots and shoot length show significant difference while the leaf number did not show significant difference in their means. Consistent with the earlier report that the number of shoots, shoot length and leave number starts declining as the concentration of BAP increased from 0.5-3.0 mg/l. It was noticed that at the BAP concentrations higher than 2.5 mg/l, shoots showed bushy feature and distorted growth due to inhibitory effect on shoot elongation and multiplication. In related studies, decline in shoot number, shoot length and leave number was reported on Awassa-83, Beletech, Adu and Barkumie and other sweet potato genotypes (Addis, 2013, Neja, 2009; Geleta and Tileye, 2011).
A different approach by Tassew (2012) has been reported for the applications of combination of the two growth regulators (BAP and GA3) instead of BAP alone that has resulted to less number of shoot per node.
Effect of IBA in vitro rooting
The highest in vitro rooting percentage (100±0.00) was observed on ½ MS media supplemented with 0.5 mg/l IBA and IBA free media. The minimum in vitro rooting percentage (33.33±0.01) was observed on MS medium supplemented with 1 mg/l IBA. However, 93.33% root formation was obtained from ex vitro rooting. The maximum ex vitro rooting response was not significantly different from maximum in vitro rooting responses (Table 2). The maximum number of roots per shoot (7.44±0.38) with average root length (6.22±0.11 cm) was found on ½ MS medium supplemented with 0.5 mg/l IBA. A minimum of 3.33±0.33 and 4.09±0.11 cm number of roots and root lengths, respectively, were observed for ½ MS medium supplemented with 1.0 mg/l IBA (Table 2). As the concentration of IBA increased, number of root and length of roots were significantly reduced. This indicates that rooting was highly influenced by the concentrations of IBA used. The ease of rooting in sweet potato is due to the presence of high endogenous auxin concentration in the explanted organ. Hence, appropriate amounts of auxin in the rooting medium are crucial for root induction. Similar result was reported by Geleta and Tileye (2011) on Awassa-83, Guntute and Awassa local sweet potato varieties.
From the rooting experiment, ex vitro could be taken as the best option that replaces in vitro root formation. It reduces the cost of the growth regulator. The ease of rooting in sweet potato is due to the presence of high endogenous auxin concentration in the explanted organ.
Roots developed through ex vitro rooting were significantly longer compared to those developed in vitro (Table 2). The highest root length (9.80±0.00 cm) was observed on ex vitro rooted shoots. The ex vitro system provides better rooting system during simultaneous rooting and acclimatization period and reduce the cost associated with growth regulators during in vitro rooting. There are a number of reports on ex vitro rooting in different crops such as Rotula (Martin, 2003), pistachio (Benmahioul et al., 2012), Agapanthus (Ponnusamy and Vanstaden, 2013), tea (Ranaweera et al., 2013), Malus zumi (Jin et al., 2008), Hagenia abyssinica (Tileye et al., 2007) and Passiflora (Mahipal et al., 2015). This is the first report on ex vitro rooting of shoots of sweet potato.
The ex vitro developed roots were non-fragile during handling (Figure 3). However, in vitro developed roots have been found to be thick, fragile and easily breakable during handling. This is due to the development of structural abnormalities in in vitro roots (Kataoka, 1994). This makes the ex vitro rooting method cost effective, time saving and more suitable for transferring into natural environment compared with the in vitro development of roots. Absences of root hairs in in vitro developed roots are also reported to affect their establishment in ï¬eld soil under commercial-scale cultivation (Debergh and Maene, 1981). This makes the ex vitro rooting method cost effective, time saving and more suitable for transferring into natural environment.
Acclimatization of plantlets
The in vitro as well as ex vitro rooted plantlets were hardened in the greenhouse. After a month of acclimatization, 84% of plantlets survived and successfully established from in vitro rooted plantlets. From the plantlets rooted in ex vitro system, 93% successfully survived and acclimatized (Figure 4). Better plantlets survivals were observed on ex vitro rooted plantlets than in vitro rooted plantlets. This is due to higher root length which playing crucial role in nutrient absorption (Kumar et al., 2014). The current results is in harmony with the finding of Berihu (2014) who reported 81.25% and 70.59 % plantlet survival, using the mixture of moist red soil, sand soil, and compost in the ratio of 1:2:1. Tassew (2012) also obtained 80% - 90% of survived plantlets after one month acclimatization.
Among BAP concentrations used for shoot initiation, 0.5 mg/l BAP was found to be the optimum concentration. The effects of BAP concentrations on shoot multiplications were also highly significant. The maximum shoot/explant (5.33 ±0.00) and shoot length (7.82±0.02 cm) were observed on MS medium supplemented with 1.0 mg/l BAP. For in vitro rooting, half strength MS medium with 0.5 mg/l IBA showed the highest value for percentages of rooting and number of root/shoot.
Although, statically no significant difference was observed among 0.5 mg/l BAP and ex vitro rooting, but higher root length response were recorded at ex vitro rooted plantlets. About 84% of the in vitro rooted plantlets were acclimatized successfully, whereas, 93% of the ex vitro rooted plantlets survived and acclimatized successfully. Therefore, 0.5 mg/l BAP for shoot initiation, 1 mg/l BAP of shoot multiplication and directly transferring to soil for rooting was recommended for micropropagation of Kulfo sweet potato variety.
The authors have declared no conflict of interests.
The authors are very grateful to the Gesha Educational Office for financial support provided to Beyene B and the Department of Plant Science and Horticulture, Jimma University for providing plant tissue culture laboratory facility. Hawassa Agricultural Research Centre is also acknowledged for providing us the sweet potato samples.
REFERENCES
|
Addis N (2013). Low cost micropropagation techniques for sweet potato (Ipomoea batatas (L.) Lam.). M.Sc. Thesis. Addis Ababa University.
|
|
|
|
Benmahioul B, Dorion N, Kaid-Harche M, Daguin F (2012). Micropropagation and ex vitro rooting of pistachio (Pistacia vera L.). Plant Cell, Tissue and Organ Culture108:353-358.
Crossref
|
|
|
|
|
Berihu M (2014). In Vitro Propagation of Sweet Potato (Ipomoea batatas (L.) Lam.) through lateral bud culture. M.Sc. Thesis. Haromaya University.
|
|
|
|
|
Dagne Y, Tewodros M, Asfaw K (2014). Development of High Yielding Taro (Colocacia esculenta L.) Variety for Mid Altitude Growing Areas of Southern Ethiopia. Journal of Plant Science 2:50-54.
|
|
|
|
|
Debergh P, Maene L (1981). A scheme for commercial propagation of ornamental plants by tissue culture. Scientia Horticulturae 14:335-345.
Crossref
|
|
|
|
|
Geleta D, Tileye F (2011). In vitro production of virus-free sweet potato (Ipomoea batatas (L.) Lam) by meristem culture and thermotherapy. Sinet: Ethiopian Journal of Science 34:17-28.
|
|
|
|
|
George E, Hall M, Deklerk G (2008). Plant tissue culture procedure-back ground. In Plant propagation by tissue culture. Springer, 3rd edition, pp. 175-205.
|
|
|
|
|
Gosukonda R, Porobodessai A, Blay E, Prakash C, Peterson C (1995). Thidiazuron-induced adventitious shoot regeneration of sweet potato (Ipomoea batatas). In Vitro Cellular & Developmental Biology-Plant 31:65-71.
Crossref
|
|
|
|
|
Jin X, Wang Y, Zhang Y, Chai T (2008). Rapid in vitro multiplication and ex vitro rooting of Malus zumi (Matsumura) Rehd. Acta Physiologiae Plantarum 30:129-132.
Crossref
|
|
|
|
|
Kataoka I (1994). Influencing of rooting substrates on the morphology of papaya root formed in vitro. Journal of Tropical Agriculture 38:251-257.
|
|
|
|
|
Khadiga G, Rasheid S, Mutasim M (2009). Effect of Cultivar and Growth Regulator on In vitro Micropropagation of Potato (Solanum tuberosum L). American-Eurasian Journal of Sustainable Agriculture 3(3): 487-492.
|
|
|
|
|
Kumar A, Mahindra P, Manoj K, Amit K, Smita S, Shekhawata N (2014). In vitro propagation and ex vitro rooting of Caralluma edulis (Edgew.) Benth and Hook. An endemic and endangered edible plant species of the Thar Desert. Scientia Horticulturae 165:175-180.
Crossref
|
|
|
|
|
Laban TF, Peace K, Robert M, Maggiore K, Hellen M, Muhumuza J (2015). Participatory agronomic performance and sensory evaluation of selected orange-fleshed sweet potato varieties in south western Uganda. Global Journal of Science Frontier Research 15:25-30.
|
|
|
|
|
Mahipal S, Shekhawat N, Kannan M, Manokari C, Ranaweera K, Ranaweera M (2015). In vitro regeneration of shoots and ex vitro rooting of an important medicinal plant Passiflora foetida L. through nodal segment cultures. Journal of Genetic Engineering and Biotechnology 13:209-2014.
Crossref
|
|
|
|
|
Martin K (2003). Rapid in vitro multiplication and ex vitro rooting of Rotula aquatic Loura hoeophytic woody medicinal plant. Plant Cell Reports 21:415-420.
Crossref
|
|
|
|
|
Neja J (2009). Effectiveness of meristem culture and chemotherapy on the production of virus free sweet potato (Ipomoea batatas (L.) Lam.). M.Sc. Thesis. Addis Ababa University.
|
|
|
|
|
Ponnusamy B, Vanstaden J (2013) Rapid in vitro micropropagation of Agapanthus praecox. Research Centre for Plant Growth and Development, School of Life Sciences, University of KwaZulu-Natal Pietermaritzburg, Scottsville, South Africa.
|
|
|
|
|
Ranaweera K, Gunasekara M, Eastward J (2013). Ex vitro rooting: Low cost micro propagation technique for tea (Camellia sinensis (L) O. Kuntz) hybrids. Scientia Horticulturae 155:8-14.
Crossref
|
|
|
|
|
SAS Inistitute (2008). SAS/STAT 9.1 User's Guide the Reg Procedure:(Book Excerpt), SAS Institute.
|
|
|
|
|
Shonga E, Gemu M, Tadesse T, Urage E (2013). Review of entomological research on Sweet potato in Ethiopia. Discourse Journal of Agriculture and Food Sciences 1:83-92.
|
|
|
|
|
Tassew G (2012). In vitro regeneration of sweet potato (Ipomoea batatas (L.) Lam.) from leaf and petiole. M.Sc. Thesis. Addis Ababa University.
|
|
|
|
|
Tekalign W, Tileye F, Girma B, (2012). Meristem culture of selected sweet potato (Ipomoea batatas L. Lam.) cultivars to produce virus-free planting material. Journal of Horticultural Science and Biotechnology 87:255-260.
Crossref
|
|
|
|
|
Tileye F, Welander M, Negash L (2007). Genetic stability, ex vitro rooting and gene expression studies in Hagenia abyssinica. Biologia plantarum 51:15-21.
Crossref
|
|
|
|
|
Tumwegamire S, Kapinga R, Rubaihayo P, LaBonte D, Grüneberg W, Burgos G, Zum Felde T, Carpio R, Pawelzik E, Mwanga R (2011). Evaluation of dry matter, protein, starch, sucrose, β-carotene, iron, zinc, calcium, and magnesium in East African sweetpotato [Ipomoea batatas (L.) Lam] germplasm. HortScience 46(3):348-357.
Crossref
|
|