ABSTRACT
Alcohol production in industry is carried out almost exclusively by means of batch or continuous fermentation processes. In both cases, cells are found spread throughout the fermenting substrate and have to go through a centrifugation cycle in order to be recovered. Investment in equipment is very high and performance is often lower than expected. According to available information, ethanol fermentation performance could be better in conditions where the cells are immobilized in continuous processes. In this experiment, it was possible to affirm, based on statistical data that fermentation with immobilized cells improves alcohol yields. Using a free cell batch fermentation process, Zymomonas mobilis reached 59.95% of the theoretical yield. Immobilized cells reached 68.53% using a batch and 74.49% using a continuous fermentation process. Under the same conditions, Saccharomyces cerevisiae reached respectively 70.03, 77.10 and 78.47% of the theoretical yield. Higher yields were achieved for both microorganisms using mixed culture fermentation, compared to pure cultures. Under the same conditions for both pure cultures, mixed cultures reached respectively 70.86, 79.07 and 80.86% of the theoretical yield. Findings suggest that association and immobilization cultures of S. cerevisiae and Z. mobilis result in better yields for batch as well as continuous fermentation processes.
Key words: Cell immobilization, mixed cultures, ethanol fermentation, Saccharomyces cerevisiae, Zymomonas mobilis.
In Brazil, research regarding ethanol fermentation was encouraged through the National Alcohol Program in the 1980s, which aimed to promote the use of ethanol as a fuel in place of petroleum. Today, the need for cleaner energy that has less impact on the environment has once again placed ethanol production on the research agenda to develop more modern technologies aimed at enhancing production. Ethanol production in Brazil has expanded continuously; according to NovaCana.com (2015a), in the period from April 2014 to March 2015, total sales of ethanol in Brazil reached 25.17 billion liters.
Most of the ethanol is produced by batch fermentation fed with yeast recycle. The remainder is produced by multi-stage continuous fermentation also with yeast recycle (NovaCana.com, 2015b).
In the batch system, a large amount of substrate is used in a fermenter and inoculated with 5 to 10% active suspension of growing yeast. When nutrients become depleted and ethanol production reaches maximum levels, the mixture is drained, the vat is washed and a new cycle begins. Currently, the fermentation cycle lasts an average of 8 to 10 h. Consequently, in batch fermentation, this interval for cleaning and yeast growth stages represents the main obstacle to more rapid fermentation. The low productivity period associated with the cyclic emptying time, cleaning and recharging the fermenter results in lower total ethanol productivity.
The continuous fermentation process has some advantages compared to batch process such as the reduction of non-productive time of cleaning, loading, unloading, etc. (Oliva-Neto et al., 2013). This process can operate continuously for longer periods and results in more uniform final products. However, the performance of continuous fermentation may be inhibited by high concentrations of ethanol.
Studies have shown that the cell immobilization method operates more efficiently in continuous processes, because it eliminates the use of filters, decanters or centrifuges to recover the cells that should be returned to the fermentation vats, resulting in better ethanol yields in experimental conditions (Black et al., 1984; Shafaghat et al., 2011).
Margaritis et al. (1983) and Behera et al. (2010a) found out that immobilized cells afforded desirable properties to the biological system which are not easily obtained in processes employing free cells. Systems with immobilized cells allow higher cell density in the bioreactor, high dilution rates without a decrease in microbial population due to the drag of the culture, and are less susceptible to the effects of inhibitory compounds and nutrient depletion.
In almost all immobilized cell systems, the presence of dissolved solids (nutrients) in the medium is concentrated in the solid-liquid interface. Thus, the immobilized cells are exposed to a higher concentration of nutrients resulting in substantial increase in reaction rates. With immobilization, the effective microorganism density is increased, resulting in differential speeds between cells and medium with consequent increase in substrate diffusion and therefore higher reaction rates. Basically, cell immobilization by entrapment is based on cell inclusion in an insoluble polymeric matrix. Entrapment has been referred to as a simple method for the immobilization of living cells and valued for continuous ethanol production.
Many substances with potential to constitute polymeric matrices have been proposed, such as calcium alginate, carrageenan, polyacrylamide, gelatin and epoxide.
A calcium alginate matrix was chosen for the present experiment. Alginate is a polymer consisting of D-mannuronic acid residues and L-glucuronic acid arranged in blocks along the chain (Margaritis et al., 1983; Covizzi et al., 2007). This polymer turns into gel in the presence of multivalent cations. The three-dimensional net of molecules formed in the gel is biochemically inert and cells can be trapped in their interstitial spaces. Alginate is the most widely used matrix for immobilizing microbial cells because it involves a simple and inexpensive method. The alginate gelling occurs rapidly in the presence of calcium ion without any changes in temperature, pH and osmotic pressure, which preserves the viability and activity of immobilized microorganisms (Kawaguti and Sato, 2008).
Regarding the biochemical process of ethanol biosynthesis, there are basic differences between yeasts and bacteria. While Saccharomyces cerevisiae ferments sucrose, glucose and fructose to produce ethanol and CO2, Zymomonas mobilis ferments the same sugars to produce ethanol, CO2, lactic acid and dextran. The formation of these products is due to the fact that bacteria do not follow the glycolytic pathway, like yeasts (Ernandes and Garcia-Cruz, 2011). In studies conducted with Z. mobilis, some advantages were observed when the aim is industrial ethanol production, highlighting high specific yields; tolerance to high ethanol concentrations; ability to grow in complete anaerobiosis, unlike yeast which requires some oxygen, especially for biosynthesis of unsaturated fatty acids (Rogers et al., 2007; Behera et al., 2010b; Bochner et al., 2010). Although, Z. mobilis shows good potential for use in ethanol fermentation, it has some limitations with respect to its fermentation capacity. Aiming to overcome these limitations and increase fermentation yields, studies were carried out using mixed inoculum of Saccharomyces uvarum IZ1904 and Z. mobilis CP4 (Morais et al., 1992). In this experiment, microorganism association increased ethanol production.
Considering the findings of other researchers who used yeasts and bactérial cells, both immobilized for ethanol production, the present study aimed, in laboratory context, to compare fermentation performance of free and immobilized cells; to compare alcohol yields in batch and continuous fermentation processes; and to evaluate possible synergistic effects of using these techniques combining S. cerevisiae and Z.mobilis.
Microorganisms
Z. mobilis (CP4-a), provided by the Institute of Antibiotics, UFPE and S. cerevisiae, Fleishmann ®.
Fermentation medium of sugarcane juice supplemented with nutrients (CSN)
Two egg whites beaten stiff were mixed in 1 L of sugarcane juice and placed in flowing steam for 15 min in autoclave. After cooling, it was filtered with cotton and diluted to 10°Brix. to 1000 ml of clarified broth, 5 g of yeast extract was added and the pH was adjusted to 5.0.
Cell immobilization method
Cell immobilization for batch fermentation
Three polypropylene bags, each containing 20 ml of 1.8% sodium alginate, were prepared. After autoclaving, 0.8 ml of Z. mobilis inoculum was added to the first bag, 0.8 ml of S. cerevisiae inoculum to the second bag and 0.4 ml of inoculum of each microorganism, forming the mixed culture, was added to the third bag. The inocula were standardized containing 109 cells/ml. The blends were dripped aseptically through semi capillary tubes with stirring in 3 beekers containing 500 ml of calcium chloride (0.05 M) solution sterilized and maintained at 4°C. Under these conditions, the beads formed measured about 2 mm in diameter and were hardened in this solution for 1 h.
Cell immobilization for continuous fermentation
Three polypropylene bags, each containing 80 ml of 1.8% sodium alginate, were prepared. After autoclaving, 3.2 ml of Z. mobilis inoculum was added to the first bag, 3.2 ml of S. cerevisiae inoculum to the second bag, and 1.6 ml of inoculum of each microorganism was added to the third bag. Each inoculum had a concentration of 109 cell/ml.
Fermentation
Batch fermentation with free cells
Z. mobilis inoculum (0.8 ml) was transferred into an Erlenmeyer flask containing 100 ml of CSN medium and incubated for 24 h. This medium was then aseptically centrifuged to recover the cells. The alcohol concentration and total reducing sugars (TRS) were quantified. The biomass obtained was again inoculated into 100 ml of new medium (CSN). The procedure was repeated 5 times totaling 120 h of fermentation with the same cells. The same methodology was applied to S. cerevisiae. For mixed cultures, 0.4 ml of each inoculum was added to the CSN medium. The inoculum was standardized with 109 cell/ml.
Batch fermentation with immobilized cells
The beads formed from 20 ml calcium alginate with Z. mobilis suspension were put into Erlenmeyer flasks containing 100 ml of CSN medium. The procedure was the same as for S. cerevisiae and mixed culture. After 24 h, the medium was drained to quantify alcohol content and TRS. To the same bottles, 100 ml of CSN medium was added. The experiment was repeated 5 times, completing 120 h of fermentation, with alginate beads containing immobilized cells.
Continuous fermentation with immobilized cells
Three continuous fermentation experiments were performed with Z. mobilis, S. cerevisiae and mixed culture in a glass column with a total volume of 143 ml. This column was filled with 80 ml alginate beads (56% of column volume), leaving 63 ml (44%) of usable volume. In the batch tests, the proportion of cells and medium was 20%. To maintain the same rate in the column, the flow was adjusted to 400 ml/24 h (16.6 ml/h). Residence time was approximately 3 h 47 min.
In all three continuous fermentation assays, the column and the vessel containing the fermentation medium were arranged in series at different heights so that the medium level could feed the column continuously.
After 24 h, the flask containing fermented medium collected continuously was replaced by a sterilized empty flask. This procedure was repeated 5 consecutive times completing 120 h of continuous fermentation. With the product, the alcohol content and TRS were quantified.
Quantification of alcohol and total reducing sugars (TRS)
The ethanol concentration was determined by gas chromatography equipment Chromatograph GC 2014 Shimadzu, Rtx®5 column. The TRS of the samples was determined by 3.5 dinitrosalicylic acid method ADNS (Miller, 1959).
Determination of fermentation yields
The yield is defined as the amount of ethanol produced in relation to the maximum ethanol that could be produced from the initial TRS. By stoichiometric reaction, 1 mole of sucrose could produce 4 moles of ethanol, so 100 g of sucrose could produce 53.8 g of ethnol if the conversion were 100%. Thus, the yields were determined by the following formulas:
EtOH max = Initial sucrose × 53.8 / 100
Where EtOHmax is the maximum production of ethanol obtained from the available sucrose (initial sucrose) and 53.8 is Gay-Lussac coefficient for sucrose.
EtOH yield (%) = Sample ethanolic concentration × 53.8 / EtOH max
% of theoretical yield = EtOH yield × 100 / 53.8
Statistical design
The statistical design was determined by analysis of variance (ANOVA) and Tukey test evaluating the averages of ethanol yields in relation to theoretical yield obtained from Z. mobilis, S. cerevisiae and mixed cultures under conditions of free cell in batch fermentation and immobilized cells in batch and continuous fermentation. Since the fermentation process began to stabilize only after 72 h, the means used for the statistical analyses were based on yields at 72, 96 and 120 h of fermentation.
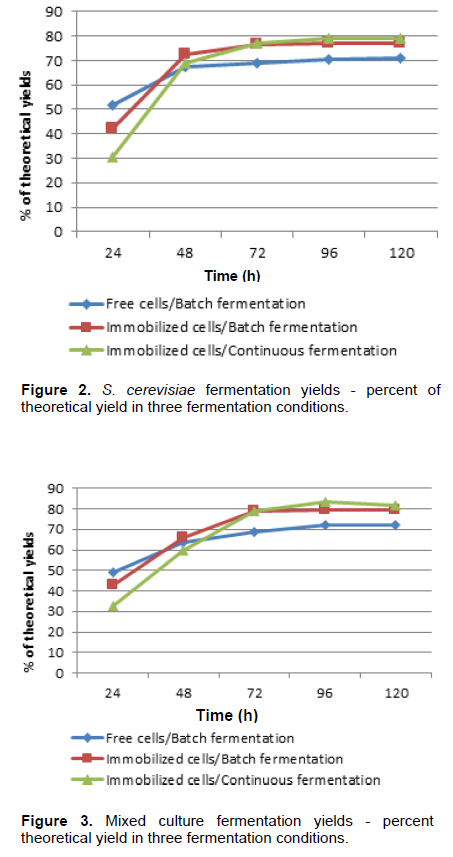
In the fermentation process, there was an adaptation of pure and mixed cultures to the condition of immobilized cells within the first 48 h. In this first stage of the experiment, there was a lag phase followed by cell growth inside the beads, an observation also reported by Duarte et al. (2013). Thus, part of sugar consumption and consequent energy production was reversed for cell reproduction. The fermentation process stabilization in three conditions occurred after 72 h. Although, the immobilized cultures delay the start of optimized fermentation, the subsequent yields indicate that immobilization benefits the fermentation process. The ethanol yield of Z. mobilis in immobilized cells and batch fermentation system, with 120 h, was about 9% higher compared to free cells system (Figure 1). After a period of 120 h, yeast in batch/immobilized system showed yield about 6% higher than fermentation with free cells (Figure 2). With regard to mixed culture with 120 h, there was a favoring of batch fermentation with immobilized cells of approximately 7% compared to batch fermentation with free cells (Figure 3). Comparison made between batch and continuous process, both with immobilized cells, showed that continuous fermentation improved fermentation process with higher ethanol production rates for all three culture conditions.
Starting with average concentration of 97.99 g/L of TRS, the ethanol concentration produced by Z. mobilis in continuous fermentation was 38.47 g/L, and in batch fermentation 35.20 g/L. The literature reports higher yields (Behera et al., 2012), but in this experiment, this was not observed. These ethanol concentrations represented 78 and 76% of theoretical yield, respectively (Figure 1). Under the same conditions, S. cerevisiae reached 42.80 and 39.87 g/L, representing 79 and 77% of theoretical yield, respectively (Figure 2). Fermentation yield for S. cerevisiae came close to yields described by Shafaghat et al. (2011) and Duarte et al. (2013). In mixed culture, the indexes were 44.13 and 40.93 g/L, representing 81 and 79% of theoretical yield, respectively (Figure 3).
The statistical design was determined by analysis of variance ANOVA (Table 1) and Tukey test (Table 2). This
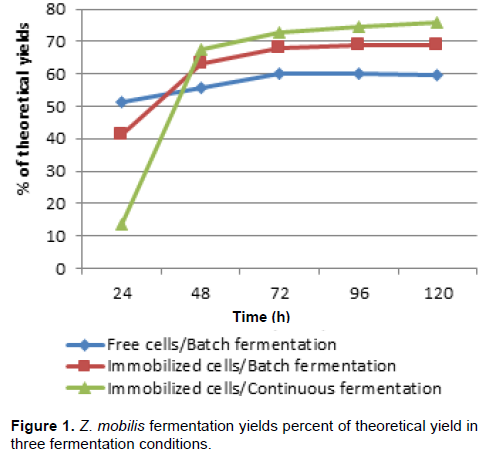
test indicated that the cultures had different behaviors according to fermentation method, making it possible to infer that the process with immobilized cells gives better yields than the process with free cells, with significant difference in 5 and 1% probability levels.
According to Tukey test, there was a significant increase in continuous process yields compared to batch process with immobilized cells, except for S. cerevisiae (Table 2).
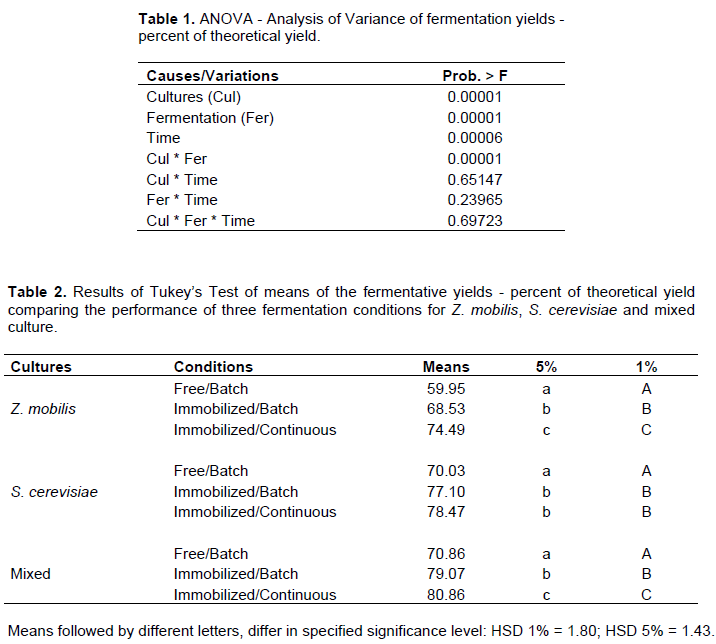
Regarding microorganism association, mixed culture showed better ethanol yields when compared with pure cultures. Z. mobilis in pure culture and in the presence of sucrose can divert part of fructose to form levan (Silbir et al., 2014). However, in the presence of S. cerevisiae, the fructose resulting from sucrose inversion could be absorbed by yeast and transformed into ethanol, leading to better yields, since some sugars would be available and not polymerized in levan form.
In batch fermentation with free cells, the mixed culture obtained a yield, at 120 h fermentation, approximately 11% greater than the culture of Z. mobilis and only 0.8% greater than S. cerevisiae culture (Figure 4). The mixed culture yield, after a period of 120 h of batch fermentation with immobilized cells, was about 10.5 and 2% higher in relation to Z. mobilis and S. cerevisiae, respectively (Figure 5). In the continuous process with immobilized cells, at 120 h, mixed culture fermentatation yields were about 6% higher than Z. mobilis and 2% higher than S.
cerevisiae (Figure 6).
The Tukey test showed significant difference in 5 and 1% probability levels, with increased fermentation yields of mixed cultures compared to pure cultures, except for the system of free cells in fermentation batch, where the yield of mixed culture showed no difference from S. cerevisiae, as shown in Table 3.
Analysis of fermentation processes showed that cell immobilization favored the production of ethanol, significantly increasing the fermentative yields when compaired with the free cells process, confirming the initial hypothesis of this work.
Continuous fermentation system increased the ethanol fermentation process, with higher production rates for all three culture conditions. However, according to theTukey test, the higher yield of S. cerevisiae in continuous fermentation compared to batch with immobilized cells was not significant.
The association of S. cerevisiae and Z. mobilis in mixed culture showed better fermentation yields in processes with immobilized cells, with differences that were statistically significant. But in free cell system this synergistic effect was not statistically significant when the performance of S. cerevisiae was compared with the The results of the experiment suggest that association and immobilization cultures of S. cerevisiae and Z. mobilis could be recommended to achieve better fermentation yields for both batch and continuous fermentation.
The authors have not declared any conflict of interests.
Financial support: CAPES.
REFERENCES
|
Behera S, Kar S, Mohanty RC, Ray RC (2010a). Comparative study of bio-ethanol production from mahula (Madhuca latifolia L.) flowers by Saccharomyces cerevisiae cells immobilized in agar agar and Ca-alginate matrices. Appl. Energy 87(1):96-100.
Crossref
|
|
|
|
Behera S, Mohanty R, Ray R (2010b). Ethanol fermentation of mahula (Madhuca latifolia) flowers using free and immobilized bacteria Zymomonas mobilis MTCC 92. Biologia 65(3):416-421.
Crossref
|
|
|
|
Behera S, Mohanty RC, Ray RC (2012). Ethanol fermentation of sugarcane molasses by Zymomonas mobilis MTCC 92 immobilized in Luffa cylindrica L. sponge discs and Ca-alginate matrices . Braz.J. Microbiol. 43(4):1499-1507.
Crossref
|
|
|
|
Black GM, Webb C, Matthews TM, Atkinson B (1984). Practical reactor systems for yeast cell immobilization using biomass support particles. Biotechnol. Bioeng. 26(2):134-141.
Crossref
|
|
|
|
Bochner B, Gomez V, Ziman M, Yang S, Brown SD (2010). Phenotype microarray profiling of Zymomonas mobilis ZM4. Appl. Biochem. Biotechnol. 161(1-8):116-123.
|
|
|
|
Covizzi LG, Giese EC, Gomes E, Dekker RF, Da Silva R (2007). Imobilização de células microbianas e suas aplicações biotecnológicas.Semina: Ciênc. Exatas Tecnológicas 28(2):143-160.
Crossref
|
|
|
|
Duarte JC, Rodrigues JA, Moran PJ, Valença GP, Nunhez JR (2013). Effect of immobilized cells in calcium alginate beads in alcoholic fermentation. AMB Express 3(1):31.
Crossref
|
|
|
|
Ernandes FMPG, Garcia-Cruz CH (2011). Uso de caldo de cana-de-açúcar para produção de levana por Zymomonas mobilis CCT 4494. Ciênc. Agrotecnologia 35:354-360.
Crossref
|
|
|
|
Kawaguti HY, Sato HH (2008). Produção de isomaltulose, um substituto da sacarose, utilizando glicosiltransferase microbiana. Quím. Nova 31(1):134.
Crossref
|
|
|
|
Margaritis A, Merchant FJ, Abbott BJ (1983). Advances in ethanol production using immobilized cell systems. Crit. Rev. Biotechnol. 1(4):339-393.
Crossref
|
|
|
|
Miller GL (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31(3):426-428.
Crossref
|
|
|
|
Morais JOF, Araújo JM, Rios MEMM, Queiroz MFV, Maia MHD, Serzedello A, Bertolin AO, Angelis DF, Buzato JB, Colabone MAP, Lima OG (1992). Aperfeiçoamento em processo de fermentação alcoólica. INPI - 845673.
|
|
|
|
NovaCana.com. (2015a). Produção de Etanol: Tecnologia praticada no Brasil.
|
|
|
|
NovaCana.com. (2015b). Números finais da safra 2014/2015 e iniciais da safra 2015/2016.
|
|
|
|
Oliva-Neto P, Dorta C, Carvalho AFA, Lima VMG, Silva DF (2013). The Brazilian technology of fuel ethanol fermentation—yeast inhibition factors and new perspectives to improve the technology. Materials and Processes for Energy: Commun. Curr. Res. Technol. Dev. 1:371-379.
|
|
|
|
Rogers PL, Jeon YJ, Lee KJ, Lawford HG (2007). Zymomonas mobilis for fuel ethanol and higher value products. In: Biofuels. Springer Berlin Heidelberg. pp. 263-288.
Crossref
|
|
|
|
Shafaghat H, Najafpour GD, Rezaei PS, Sharifzadeh-Baei M (2011). Ethanol production with natural carbon sources in batch and continuous fermentation using free and immobilized Saccharomyces cerevisiae. J. Sci. Ind. Res. 70:162-169.
|
|
|
|
Silbir S, Dagbagli S, Yegin S, Baysal T, Goksungur Y (2014). Levan production by Zymomonas mobilis in batch and continuous fermentation systems. Carbohydr. Polym. 99:454-461.
Crossref
|