ABSTRACT
In this study, three Bacillus subtilis (B7, B9 and B3) and one Bacillus amyloliquefaciens (I8) strains previously selected as potential starter cultures were cultivated on boiled dehulled African locust bean seeds to produce ready to use ferments. These ferments were then used to produce soumbala. The microbial load changes during ferments production were evaluated. Biochemical and microbiological characteristics of the obtained soumbala were also determined using standard methods. Variable growth ability on the carrier material was observed for the tested starters with Bacillus loads ranging between 8.21 and 10.37 Log CFU/g in the ferments. The highest microbial counts were observed for ferments prepared with the starters B9 and B7. These ferments also demonstrated the strongest fermentation capacity of soumbala. The ferment-based dried soumbala had a pH and moisture ranging from 7.17 to 7.37 and 5.67 to 8.46%, respectively. On dry matter (DM) basis, it contained 1.77 to 2.11% of ashes, 41 to 43% of proteins, 37 to 40% of fat and 13 to 15% of carbohydrates. Soumbala prepared with the starter B7 showed the highest content in valine, isoleucine, leucine, phenylalanine, tyrosine and proline.
Key words: African locust bean, starter culture, Bacillus, fermentation, soumbala, carrier.
Fermented food condiments obtained by the fermentation of proteagenous seeds, are well appreciated in Africa for their high nutritional value and organoleptic properties. In Burkina Faso, the well-known of these fermented food condiments is soumbala, obtained by spontaneous alkaline fermentation of African locust bean (Parkia biglobosa) seeds (Parkouda et al., 2009). Soumbala is also well known and used in Côte d’Ivoire, Guinea, and Mali. It is known under different names such as dawadawa/iru in Nigeria and Ghana (Onzo et al., 2014; Ajavi et al., 2015), afitin/sonru/iru in Benin (Azokpota et al., 2006) and nététu in Senegal (N'Dir et al., 2000).
Soumbala is an affordable source of proteins (34-42%), fat (21-37%), carbohydrates (15-17%), minerals (calcium, phosphorus and iron), vitamins (B1 or thiamine, B2 or riboflavin and PP or niacin) and essential amino acids (Ouoba et al., 2003b) for low income inhabitants. In addition, the production of soumbala constitutes an income generator for producers who are generally illiterate women.
The production of soumbala includes successive cleaning of the seeds, a first cooking which often lasts more than 24 h, a dehulling of the cooked seeds, a second cooking which lasts between 1 and 2 h and then a spontaneous fermentation of 48-72 h (Sawadogo-Lingani et al., 2003). Bacillus subtilis group species were identified as the dominant Bacillus involved in the spontaneous fermentation of soumbala (Ouoba et al., 2004). Despite increasing consumption today in Burkina Faso, soumbala still faces competition from imported seasonings. This is partly due to the use of unsuitable fermentation methods in the production of traditional soumbala leading to a product with poor organoleptic and sanitary quality resulting sometimes in the presence of pathogenic bacteria and biogenic amines (Parkouda et al., 2010).
Several studies carried out on soumbala and other fermented condiments of Burkina Faso provided evidence on how to isolate and characterize some Bacillus species with potential uses as starter cultures in controlled fermentation to improve its hygienic, nutritional and organoleptic quality (Ouoba, 2003; Kaboré, 2012; Compaoré, 2013). However, the form in which these potential starter cultures can be easily transferred to soumbala production units has not been proposed yet. As a consequence, soumbala processing units are still producing soumbala in a traditional way with uncontrolled fermentation. The objectives of this study were, therefore on one hand to assess the possibility of using the dehulled seeds of African locust bean as local carrier material for the transfer of starter cultures of Bacillus spp. to soumbala production units, and on the other hand to compare the biochemical and microbiological characteristics of soumbala prepared with starter cultures used in single or in combination.
African locust bean seeds
African locust bean seeds were purchased with a soumbala producer in Ouagadougou, Burkina Faso, stored in polypropylene bags and kept in the pilot plant of Département Technologie Alimentaire (CNRST/IRSAT/DTA) at room temperature.
Microorganisms
The starter cultures used in this study included two strains of B. subtilis (B7 and B9) isolated from soumbala, one strain of Bacillus amyloliquefaciens (I8) isolated from bikalga (fermented seeds of Hibiscus sabdariffa) and one strain of B. subtilis (B3) originating from maari (fermented seeds of Adansonia digitata). These strains were identified based on molecular methods (Rep-PCR, ITS-PCR, M13-PCR, 16S rRNA and gyrB gene sequencing) and selected as starter cultures in previous studies based on their technological properties among other proteolytic, saccharolytic, lipolytic and antimicrobial activities (Ouoba et al., 2003a, 2003b, 2007; Kaboré et al., 2012; Compaoré et al., 2013b). All the strains were kindly provided by the laboratory of microbiology of CNRST/IRSAT/DTA where they were stored in a -80°C freezer.
METHODS
Preparation of the carrier material
African locust bean seeds were first dried and cleaned by winnowing to remove light impurities. They were then dehulled using a mechanical dehuller (prototype CNRST/IRSAT, Ouagadougou, Burkina Faso, 1997). Following dehulling, the cotyledons were separated from the hulls by winnowing and manual sorting. The cotyledons were then collected for use as carrier for the production of the ferments.
Preparation of the inocula
The stock cultures were sub-cultured in Brain Heart Infusion (BHI) agar (Liofilchem, 610007, Italy) and incubated for 24 h at 37°C. From BHI agar plates, the Bacillus strains were sub-cultured for 18 h at 37°C in 10 ml of BHI broth (Liofilchem, 610008, Italy). After incubation, the cultures were centrifuged at 5 000 g for 10 min and the pellet resuspended in 5 ml of sterile diluent containing 8.5 g/l NaCl and 1.5 g/l peptone (Difco 218971, Becton Dickinson & Co, Sparks, MD, USA). The number of cells was then estimated by microscopy using a counting chamber (Neubauer, Wertheim, Germany) and dilutions were made in sterile diluent to obtain a rate of inoculation of 105 - 106 cells/ml.
Four different inocula were prepared: Inoculum of B. subtilis B7, inoculum of B. subtilis B9, inoculum of B. subtilis B3 and inoculum of B. amyloliquefaciens I8.
Cultivation of the starter cultures on the carrier material
The African locust bean cotyledons were weighed and washed before being boiled for 6 h. After cooking and draining, the cotyledons were distributed (500 g) in baskets and autoclaved at 121°C for 20 min. After cooling at 45 to 50 °C, each basket was inoculated with each inoculum (2% v/w) in single and left to ferment for 48 h at room temperature (35 - 38°C). One non-inoculated basket served as control.
The fermented cotyledons from each basket were dried in an oven at 60 to 65°C for 24 h before being aseptically ground using a blender of mark XPREP (Model MX1200XT11CE, USA). The resulting powder was aseptically packaged (5 g) in sterile plastic bags and stored at room temperature. Four ferments FB7, FB3, FI8 and FB9 in powder form were then prepared from inoculum of B. subtilis B7, B. subtilis B3, B. amyloliquefaciens I8 and B. subtilis B9, respectively. Samples were collected after autoclaving, after inoculation (0 h of fermentation), at the end of the fermentation (48 h) and after the grinding of dried product to determine pH and growth of Bacillus for each single starter culture fermentation batch. The experiment was performed on three separate occasions and 16 samples were taken at each trial. In total, 48 samples were collected for microbiological analyses.
Production of soumbala with the ferments
The production of soumbala was carried out with non-dehulled African locust bean seeds following the traditional processing described by Sawadogo-Lingani et al. (2003) with slight modifications as follows: the seeds were cleaned, cooked for 18 h and dehulled manually with mortar and pestle; the dehulled seeds were cooked again for 1 h, drained, distributed in baskets, autoclaved at 121°C for 20 min and cooled to 45 to 50°C. Seven parallel fermentation batches were prepared as follows: the batches 1, 2, 3 and 4 containing 1 kg of sterilized cotyledons each, were inoculated with 5 g of each single ferment (FB7, FB9, FB3 and FI8); the batches 5, 6 and 7 containing 2 kg of sterilized cotyledons each were inoculated with 10 g of mixed ferments (FB7+FB3, FB7+FI8 and FB7+FB9). The batches were then left to ferment for 48 h at room temperature (35 - 38°C). Traditional spontaneously fermented soumbala was prepared according to Sawadogo-Lingani et al. (2003) to serve as a control (batch 8). After the fermentation, fermented cotyledons were sun-dried and kept in a dry place. Samples were collected at 0 h, at the end of the fermentation (48 h) and after drying. The different types of soumbala produced were:
(1) Soumbala with single ferment: SB7, SB3, SI8, and SB9
(2) Soumbala with mixed ferments: SB7 + B3, SB7 + I8, SB7 + B9
(3) Spontaneously fermented soumbala: SN.
The experiment was conducted in triplicate and 24 samples were taken at each assay. In total 72 samples were collected for microbiological analyses. Physicochemical analyses were performed only on the final dried products (24 samples).
Microbiological analyses
For all samples, 10 g were aseptically homogenized with 90 mL of sterile diluent by using a stomacher (Stomacher 400 lab blender, England) at normal speed for 2 min to obtain 10-1 dilution (ISO 6887-1, 2017). Serial dilutions were made from the homogenate using 9 mL sterile diluent. From appropriate ten-fold dilutions, Bacillus strains were enumerated by pour plate technique using BHI Agar incubated aerobically at 37°C for 72 h. After incubation, plates with 15 to 300 colony forming units (CFU) were counted (ISO 4833, 2003) and results expressed as Log CFU/g.
Biochemical analyses
Ten grams of sample were homogenized with 20 mL of distilled water in a stomacher bag for 1 min at normal speed. The pH of the homogenate was determined using an electronic pH meter (Hanna, Romania) calibrated with standard buffer solutions pH 4.0 and 7.0.
Moisture content was determined by drying the sample at 105 ± 2°C for 12 h according to ISO 712 (2009); total ash content was determined by incineration in a muffle furnace (Nabertherm, Germany) at 550°C for 4 h, according to ISO 2171 (2007); crude protein content (N×6.25) was determined by the Kjeldahl method after acid digestion (AFNOR NF V03-050, 1970); crude fat content was determined with Soxhlet apparatus using n-hexane according to ISO 659 (1998). Total carbohydrates content was determined by spectrophotometric method at 510 nm using sulfuric orcinol as reagent (Montreuil and Spik, 1969).
For amino acids profile determination samples were first defatted using Soxhlet method (ISO 659. 1998). The amino acid profile was carried out by high performance liquid chromatography (HPLC) using Waters PICO-TAG method (Kristofferson, 2011) which consists of three steps: hydrolysis of samples, sample derivatization pre-column and HPLC-reverse phase analysis. The identification and determination of the concentrations of the different amino acids were done from the Empower software by comparing retention times obtained with retention times of the standards.
Statistical analysis
All the data were submitted to Analysis of Variance (ANOVA) with the statistical software XLSTAT-Pro 7.5.2 and the means were compared using a Fischer test for post-hoc comparisons with a probability level p Ë‚ 0.05. The curves and the standard deviation were obtained using Microsoft Excel 2007.
Microbial growth during the production of ferments
No microbial growth was observed after autoclaving of African locust bean cotyledons for all samples (results not shown). Figure 1 shows the growth ability of the different starter cultures inoculated in single in the cooked dehulled African locust bean seeds. At the onset of the fermentation (t = 0 h), Bacillus counts ranged between 4.33 and 4.87 Log CFU/g. At the end of the fermentation (t = 48 h), a significant increase of the Bacillus load was observed in all samples with values ranging between 7.11 and 9.70 Log CFU/g. However, the starter cultures showed variable ability to grow in the cooked dehulled seeds of P. biglobosa. Bacillus amyloliquefaciens I8 (originating from bikalga) counts were the lowest, followed by B. subtilis B3 (originating from maari). On the contrary, B. subtilis B7 and B. subtilis B9 (originating from soumbala) yielded the highest counts. In the final dried and ground products (ferments), the Bacillus counts had increased to between 8.21 and 10.37 Log CFU/g and the highest microbial counts were observed for ferments prepared with B. subtilis B9 and B7.
Microbial growth during controlled fermentation of soumbala using single and mixed ferments
The growth of Bacillus ferments inoculated in single during the production of soumbala is as shown in Figure 2. At the beginning of the fermentation, soumbala produced with single ferment (SB7, SI8, SB3 and SB9) had a Bacillus load between 6.74 and 7.66 Log CFU/g whereas spontaneous soumbala had a load of 2.86 Log CFU/g. At the end of the fermentation (48 h), the Bacillus loads increased in all types of soumbala and ranged between 7.92 and 9.54 Log CFU/g. The increase of Bacillus count was also observed in final dried products between 8.38 and 10.11 Log CFU/g. The highest Bacillus counts were obtained with ferments of B. subtilis B9 (10.11 Log CFU/g) and B. subtilis B7 (9.84 Log CFU/g). Regarding the soumbala obtained by spontaneous fermentation (SN), its Bacillus load after drying was 8.93 Log CFU/g.
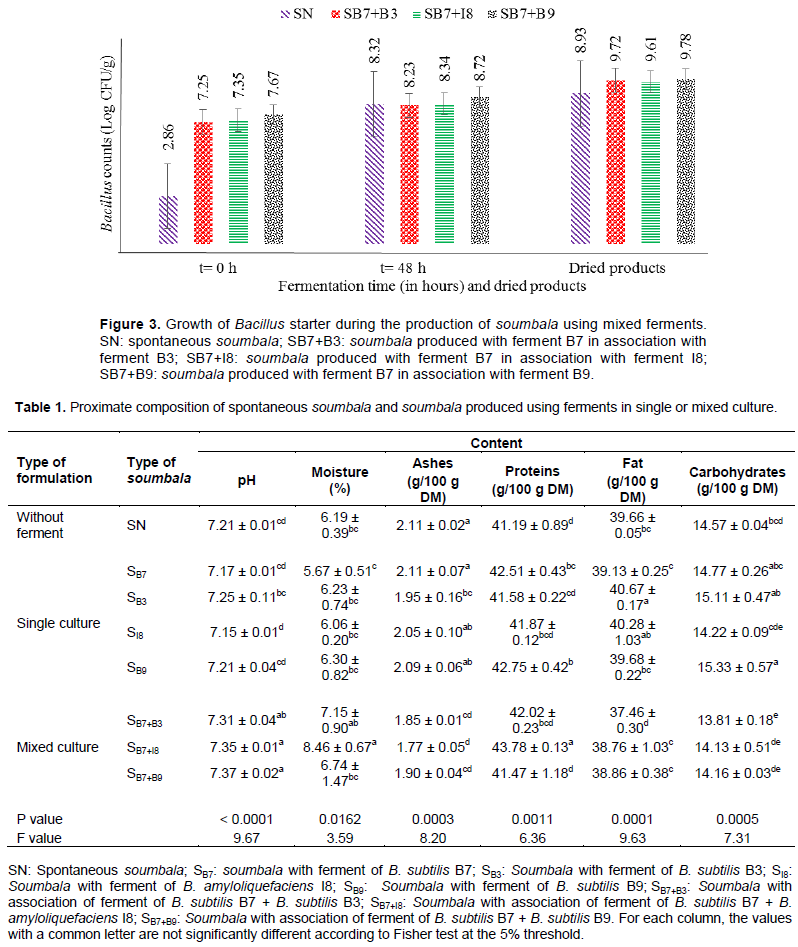
Figure 3 shows the growth capacity of the Bacillus ferments used in mixture during the controlled fermentation of soumbala. Used in mixture, the starter cultures’ loads increased from 7 Log CFU/g at the onset of the fermentation to 8 Log CFU/g at the end of the fermentation, while for spontaneous fermentation, microbial population increased from 2 to 8 Log CFU/g. After drying, the Bacillus counts were between 9.61 and 9.78 Log CFU/g for soumbala produced with ferments and 8.93 Log CFU/g for spontaneous soumbala.
Proximate composition of soumbala produced with ferments of starter cultures
Results from Table 1 show the proximate composition of the different samples of soumbala. The pH of soumbala produced with ferments of starter cultures varied from 7.17 ± 0.01 to 7.25 ± 0.11 for single culture soumbala and from 7.31 ± 0.04 to 7.37 ± 0.02 for mixed cultures soumbala. However, soumbala from spontaneous fermentation had a pH of 7.21 ± 0.01. The lowest pH value (7.15 ± 0.01) was obtained with single starter culture B. amyloliquefaciens I8 (originating from bikalga) while the highest pH (7.37 ± 0.02) was recorded with the combination of B. subtilis B7 and B9 (originating from soumbala). There was no significant difference (p ˃ 0.05) between the pH of the different soumbala produced using combined starter cultures. The moisture content of controlled fermented dried soumbala ranged between 5.67 ± 0.51 and 8.46 ± 0.67% whereas the spontaneous dried soumbala showed a moisture content of 6.19 ± 0.39%. Dried soumbala obtained with mixed starter culture SB7+I8 showed the highest moisture content (8.46 ± 0.67%). Single culture inoculated soumbala and mixed culture inoculated soumbala ash content ranged from 1.95 ± 0.16 to 2.11 ± 0.07%/DM and 1.77 ± 0.05 to 1.90 ± 0.04%/DM, respectively. Meanwhile, ash content of spontaneous soumbala was 2.11 ± 0.02%/DM. Soumbala obtained with a mixed culture of B. subtilis B7 and B. amyloliquefaciens I8 showed the lowest ash content (1.77 ± 0.05%/DM). Analyses showed that there was no significant difference (p ˃ 0.05) between ash content of spontaneous soumbala and soumbala from starter B7 (SB7), I8 (SI8) and B9 (SB9). Protein content varied between 41 and 43%/DM with spontaneous soumbala giving the weakest rate (41.19 ± 0.89%/DM) compared to mixed culture soumbala (SB7+I8) which gave the highest rate (43.78 ± 0.13%/DM). There was significant difference (p Ë‚ 0.05) between protein contents of all samples of soumbala. Regarding the crude fat content, it ranged from 37.46 ± 0.30%/DM (obtained with SB7+B3) to 40.67 ± 0.17%/DM (obtained with SB3) for soumbala produced with starter cultures and was 39.66 ± 0.05%/DM for spontaneously fermented soumbala (SN). Fat content of SB7+B9, SB7+I8 and SB7 was not significantly different (p ˃ 0.05). Likewise, there was no significant difference (p ˃ 0.05) between fat content of SN and SB9. The soumbala that was fermented using the ferment B9 had the highest total carbohydrates value (15.33 ± 0.57 %/DM), while the combination of ferments B7 and B3 gave the lowest content (13.81 ± 0.18%/DM). SN had a content of total carbohydrates of 14.57 ± 0.04%/DM.
Amino acid profiles of soumbala produced with ferments of starter cultures
Amino acid profiles (in g/100 g DM) of soumbala produced with ferments of Bacillus starter as well as soumbala from spontaneous fermentation are presented in Table 2. The different soumbala presented variable content in essential amino acids. The highest contents in valine (1.038), leucine (1.138), isoleucine (0.772), phenylalanine (0.722), tyrosine (1.064) and proline (0.641) were obtained with soumbala fermented using the ferment produced with the starter B7. However, these amino acids were observed in low concentrations in soumbala produced with the combination of starter cultures B7 and B9. The soumbala SI8 presented the lowest content in histidine (0.102) while the highest content was observed for soumbala SB9 (0.208). Threonine, methionine and alanine were found in highest concentrations in soumbala SB7+I8 with respective values of 0.109, 0.077 and 0.506. Regarding lysine, the highest content was obtained with SB7 (0.791) while the lowest content was found in SI8 (0.520).
The increase of Bacillus loads during the production of ferments indicates that the starter cultures used in the study are able to use African locust bean cooked cotyledons as substrate for their growth. However, the fermentation capacity varied among the strains. The highest loads observed with the starter B7 and B9 are probably due to the fact that these strains were previously isolated from the fermentation of the same substrate and are therefore more able to use this substrate for their growth. Indeed, the autochthonous character of these starters gives them a better implantation during the fermentation process (Fessard, 2017). The low concentrations of Bacillus in the ferments prepared with starter cultures B3 and I8 may be explained by their non-autochthonous character. Therefore, African locust bean seeds cotyledons may not be a favorite substrate for their growth.
The Bacillus loads (9.63 - 9.70 log CFU/g) found in the ferments prepared with starter cultures B7 and B9 were close to those of Agbobatinkpo et al. (2012) who also found Bacillus loads of 9.7 log CFU/g in yanyanku and ikpiru, two food additives (obtained by the fermentation of H. sabdarifa seeds) used in Benin for the fermentation of African locust bean seeds into sonru and iru. In addition, during the controlled fermentation of Afitin with B. subtilis starter cultures, the maximum load of Bacillus after fermentation was 9.5 log CFU/g (Ahonoukoun, 2014).
The increase in Bacillus load during the controlled fermentation of soumbala with the ferments demonstrates the fermentation capacity of these ferments. However, ferments produced with starter cultures B7 and B9 demonstrated the strongest fermentation capacity, with the highest loads when used in monoculture (9.84-10.11 Log CFU/g) and in mixed culture (9.79 Log CFU/g). Similar range of bacterial counts in soumbala or similar products have been reported previously (Sawadogo/ lingani et al., 2003; Parkouda et al., 2009; Amoa-Awua et al., 2014; Ajavi et al., 2015; Guissou et al., 2020).
In this study, the pH of soumbala produced with ferments of starter cultures was alkaline, like that of traditional spontaneous soumbala. This result is in agreement with those recorded for similar African fermented condiments by other authors (Azokpota et al., 2006; Akabanda et al., 2018; Mohammadou et al., 2018; Ibrahim et al., 2018). This alkaline pH is due to the proteolytic activity of the fermenting microorganisms, which degrade proteins and release ammonia in the medium (Mohammadou et al., 2018). The results reported here corroborate those of Agbobatinkpo et al. (2012) in Benin, during the study of the fermentation ability of yanyanku and ikpiru, who found an average pH ranging from between 7.1 and 7.3 for African locust bean cotyledons fermented with or without additives. However, Sawadogo et al. (2003) and Guissou et al. (2020) during spontaneous fermentation of P. biglobosa seeds to produce soumbala found lower pH values in the dried products. The low water content observed in the various soumbala would promote their conservation (Ajavi et al., 2015).
The content of ashes obtained for the different soumbala (1.77 - 2.11%) was lower than those found by Agbobatinkpo et al. (2012) which were 2.6 to 3.2%. This difference could be explained by the addition of ash solution during the preparation of the additives yanyanku and ikpiru used for the fermentation of P. biglobosa seed-based condiments in Benin, or by the difference in ash content of the seeds used in each country. The spontaneous soumbala as well as soumbala produced with ferments of starter cultures also presented lower concentrations of ashes compared to the results recently presented by Guissou et al. (2020).
The protein levels obtained in this study were higher than those obtained for sonru and iru fermented with yanyanku and ikpiru additives, which average was 35% (Agbobatinkpo et al., 2012). The variation of the protein contents may be due to the proteolytic activity of the fermenting strains (Mohammadou et al., 2018) and also to the difference in the physicochemical composition of African locust bean seeds according to the localities. Results demonstrated that controlled fermented soumbala as well as spontaneous fermented soumbala were rich in proteins (> 40%). Therefore, soumbala could be a source of protein that could help poor population to meet their requirement for this nutrient, particularly in developing countries. High amount of protein was also noted for other alkaline fermented products and was related to Bacillus counts (Terlabie et al., 2006; Mohammadou et al., 2018).
The different soumbala prepared with the ferments of Bacillus spp. presented interesting fat contents (37 - 40%). The fat content of soumbala prepared using single ferment is comparable to that reported by Guissou et al. (2020), which was 40.47%. The carbohydrate contents found are also in agreement with that reported by Guissou et al. (2020).
Results showed that soumbala produced with ferments of Bacillus spp. contained more essential amino acids than the traditional spontaneous soumbala. Soumbala produced using the starter culture B7 had the highest levels of valine, leucine, isoleucine and phenylalanine. Similar results were previously obtained by Ouoba et al. (2003b) in soumbala produced by controlled fermentation using the same B. subtilis as starter culture. As reported by the same authors (Ouoba et al., 2003b), it was also found that soumbala produced with starter culture B9 contained high content of histidine compared to soumbala produced with starter culture B7.
The presence of high amounts of lysine is particularly interesting because lysine is a limiting amino acid in cereals and seeds that constitute the staple diet of the majority of African populations (Diawara et al., 2004). The soumbala produced with this starter culture could then be used to fortify foods. The presence of non-essential amino acids such as tyrosine, proline and glycine at significant content in certain samples is also of interest since these amino acids could be essential in some human physiological circumstances (Ouoba et al., 2003b). Variable concentrations of amino acids in African fermented condiments have been reported in other studies (Parkouda et al., 2015; Akabanda et al., 2018; Ibrahim et al., 2018).
In the present study, four Bacillus strains (B. subtilis B7, B. subtilis B9, B. subtilis B3 and B. amyloliquefaciens I8) previously isolated from spontaneous fermentation of three different condiments and selected as starter cultures were successfully developed on dehulled African locust bean seeds used as carrier material to produce ferments. These ferments have been used separately or in combination to control the fermentation of African locust bean seeds into soumbala, which present interesting microbiological and nutritional characteristics. The obtained results indicate that dehulled African locust bean seeds are a promising carrier material for the transfer of Bacillus starter cultures to soumbala production units. These results may help to standardize the soumbala production process as well as its quality. However, further investigations need to be performed to evaluate the performance of these ferments in real environment and assess their stability during storage.
The authors have not declared any conflict of interests.
The authors are grateful to the Ministry of Foreign Affairs of Denmark (Danida) for funding through the project “Preserving African food microorganisms for Green Growth” (project number DFC No. 13-04KU). The authors would like to appreciate all the soumbala producers involved in the study for their collaboration.
REFERENCES
|
Agbobatinkpo PB, Dabadé SD, Fabrice L, Akissoé N, Azokpota P, Hounhouigan JD (2012). Softening effect of Ikpiru and Yanyanku, two traditional additives used for the fermentation of African Locust Bean (Parkia biglobosa) seeds in Benin. International Journal of Biological and Chemical Sciences 6(3):1281-1292.
Crossref
|
|
|
|
Akabanda F, Parkouda C, Suurbaar Jennifer, Donkor A-M, Owusu-Kwarteng J (2018). Effect of mechanical dehulling on microbiological characteristics and chemical changes during processing of Parkia biglobosa seeds into dawadawa, a West African alkaline fermented condiment. Journal of Ghana Science Association 17(2):12-19.
|
|
|
|
|
Ahonoukoun MZ (2014). Etude comparative de la qualité microbiologique de l'afitin à base des graines de néré (Parkia biglobosa) obtenu en fermentation spontanée et avec des souches pures de B. subtilis. Mémoire de Master Professionnel. Université d'Abomey-Calavi (UAC), Bénin 80 p.
|
|
|
|
|
Ajavi OA, Akinrinde IM, Akinwunmi OO (2015). Towards the development of shelf stable 'iru' (Parkia biglobosa) condiment bouillon cubes using corn, cassava and potato starch extracts as binders. Nigerian Food Journal 33:67-72.
Crossref
|
|
|
|
|
Amoa-Awua WK, Awusi B, Owusu M, Appiah V, Ofori H, Thorsen L, Jespersen L (2014). Reducing the atypical odour of dawadawa: Effect of modification of fermentation conditions and post-fermentation treatment on the development of the atypical odour of dawadawa. Food Control 42:335-342.
Crossref
|
|
|
|
|
Association Française de Normalisation (AFNOR), NF V03-050 (1970). Directives générales pour le dosage de l'azote avec minéralisation selon la méthode de Kjeldahl 8 p.
|
|
|
|
|
Azokpota P, Hounhouigan DJ, Nago MC (2006). Microbiological and chemical changes during the fermentation of African locust bean Parkia biglobosa, to produce afitin, iru and sonru, three traditional condiments produced in Benin. International Journal of Food Microbiology 107:304-309.
Crossref
|
|
|
|
|
Compaoré CS (2013). Caractérisation de Bacillus ssp. En vue de la sélection cultures starters pour une fermentation contrôlée du Bikalga (Graines fermentées d'oseille). Thèse de Doctorat de l'université polytechnique de Bobo-Dioulasso, Burkina Faso 170 p.
|
|
|
|
|
Compaoré CS, Nielsen DS, Sawadogo-Lingani H, Berner TS, Nielsen KF, Adimpong DB, Diawara B, Ouedraogo GA, Jakobsen M, Thorsen L (2013b). Bacillus amyloliquefaciens ssp. Plantarum strains as potential protective starter cultures for the production of Bikalga, an alkaline fermented food. Journal of applied microbiology 115:133-146.
Crossref
|
|
|
|
|
Diawara B, Ganou L, Sawadogo-Lingani H (2004). Composition et valeur nutritionnelle du soumbala. In Valorisation technologique et nutritionnelle du néré ou Parkia biglobosa (Jacq.) Benth : une espèce agro forestière, Diawara B. and Jakobsen M. (eds.), DANIDA-KVLCNRST/IRSAT pp. 99-106.
|
|
|
|
|
Fessard A (2017). Recherche de bactéries lactiques autochtones capables de mener la fermentation des fruits tropicaux avec une augmentation de l'activité antioxydante. Thèse de Doctorat de l'Université de la Réunion 192 p.
|
|
|
|
|
Guissou AWDB, Parkouda C, Coulibaly KA, Traoré K, Oboulbiga EB, Savadogo A (2020). Fermentation Effect on the Nutrient and Antinutrient Composition of Senegalia macrostachya and Parkia biglobosa Seeds: A Comparative Study. Food and Nutrition Sciences 11:726-740.
|
|
|
|
|
Ibrahim AD, Dandare SU, Mukhtar Sa'adat I, Adamu SA, Jumare Fatima I, Shinkafi SA (2018). Towards an efficient starter culture to produce dawadawa botso: a traditional condiment produced by fermentation of Hibiscus sabdariffa seeds. International Journal of Biological and Chemical Sciences 12(2):636-649.
Crossref
|
|
|
|
|
Kaboré D, Thorsen L, Nielsen DS, Berner TS, Sawadogo-Lingani H, Diawara B, Dicko M, Jakobsen M (2012). Bacteriocins formation by dominant aerobic sporeformers isolated from traditional maari. International Journal of Food Microbiology 154:10-18.
Crossref
|
|
|
|
|
Kaboré D (2012). Etude des potentialités métaboliques des bactéries dominantes isolées du Maari (graines fermentées de baobab) en vue d'une production contrôlée. Thèse de Doctorat Unique. Université de Ouagadougou, Burkina Faso 107 p.
|
|
|
|
|
Kristofferson TL (2011). Amino acid analysis of savannah tree seeds. Danish technological institute 31 p.
|
|
|
|
|
Mohammadou BA, Mbofung CM, Mounier J, Coton E (2018). Use of selected Bacillus spp. strains for directed fermentation of Hibiscus sabdariffa seeds into Mbuja. Asian Food Science Journal 2(2):1-9.
Crossref
|
|
|
|
|
Montreuil J, Spik G (1969). Micro Dosage des Glucides. I/ Méthodes Colorimétriques de Dosage des Glucides Totaux. Faculté des Sciences Université de Lille : Lille, France.
|
|
|
|
|
N'Dir B, Lognay G, Wathelet B, Cornelius C, Marlier M, Thonart P (2000). Composition chimique du nététu, condiment alimentaire produit par fermentation des graines du caroubier africain Parkia biglobosa (Jacq.) Benth. Biotechnology Agronomy Society and Environment 4(2):101-105.
|
|
|
|
|
Norme internationale ISO 712 (2009). Céréales et produits céréaliers. Détermination de la teneur en eau. Méthode de référence 17 p.
|
|
|
|
|
Norme internationale ISO 2171 (2007). Céréales, légumineuses et produits dérivés-Dosage du taux de cendres par incinération. 4ed., 11 p.
|
|
|
|
|
Norme internationale ISO 659 (1998). Détermination de la teneur en matière grasse selon la méthode d'extraction par Soxhlet 13 p.
|
|
|
|
|
Norme internationale ISO 6887-1 (2017). Microbiologie de la chaîne alimentaire-Préparation des échantillons, de la suspension mère et des dilutions décimales en vue de l'examen microbiologique-Partie 1 : règles générales pour la préparation de la suspension mère et des dilutions décimales 28 p.
|
|
|
|
|
Norme internationale ISO 4833 (2003). Microbiologie des aliments. Méthode horizontal pour le dénombrement des micro-organismes- Technique de comptage des colonies à 30°C 9 p.
|
|
|
|
|
Onzo CF, Azokpota P, Pierre Agbani P, Gbaguidi F, Hounhouigan JD, Kossou D (2014). Caractéristiques physico-chimiques, et toxicité des espèces végétales utilisées comme emballages alimentaires en Afrique de l'Ouest. International Journal Biological and Chemical Sciences 8(4):1504-1516.
Crossref
|
|
|
|
|
Ouoba LII (2003). Caractérisation génotypique, biochimique et activité antimicrobienne de souches de Bacillus spp. en vue de la mise au point de starters pour la production contrôlée du Soumbala. PhD Thesis. Université de Ouagadougou, Burkina Faso 125 p.
|
|
|
|
|
Ouoba LII, Cantor M, Diawara B, Traore A, Jakobsen M (2003a). Degradation of African locust bean oil by Bacillus subtilis and Bacillus pumilus isolated from soumbala, a fermented African locust bean condiment. Journal of Applied Microbiology 95(4):868-873.
Crossref
|
|
|
|
|
Ouoba LII, Rechinger K, Barkholt V, Diawara B, Traore A, Jakobsen M (2003b). Degradation of proteins during the fermentation of African locust bean (Parkia biglobosa) by strains of Bacillus subtilis and Bacillus pumilus for production of Soumbala. Journal of Applied Microbiology 94(3):396-402.
Crossref
|
|
|
|
|
Ouoba LII, Diawara B, Amoa-Awua, WK, Traore AS, Lange-Møller P (2004). Genotyping of starter cultures of Bacillus subtilis and Bacillus pumilus for fermentation of African locust bean (Parkia biglobosa) to produce Soumbala. International Journal of Food Microbiology 90:197-205.
Crossref
|
|
|
|
|
Ouoba LII, Diawara B, Jespersen L, Jakobsen M (2007). Antimicrobial activity of Bacillus subtilis and Bacillus pumilus during the fermentation of African locust bean (Parkia biglobosa) for Soumbala production. Journal of Applied Microbiology 102:963-970.
|
|
|
|
|
Parkouda C, Nielsen DS, Azokpota P, Ouoba LII, Amoa-Awua WK, Thorsen L, Hounhouigan JD, Jesen JS, Tano-Debrah K, Diawara B, Jakobsen M (2009). The Microbiology of Alkaline-Fermentation of Indigenous Seeds Used a Food Condiment in Africa and Asia. Critical Reviews in Microbiology 35:139-156.
Crossref
|
|
|
|
|
Parkouda C, Thorsen L, Compaoré CS, Nielsen DS, Tano-Debrah K, Jensen JS, Diawara B, Jakobsen M (2010). Microorganisms associated with maari, a baobab seed fermented product. International Journal of Food Microbiology 142:292-301.
Crossref
|
|
|
|
|
Parkouda C, Ba F, Ouattara L, Tano-Debrah K, Diawara B (2015). Biochemical changes associated with the fermentation of baobab seeds in Maari: An alkaline fermented seeds condiment from western Africa. Journal of Ethnic Foods 2(2):58-63.
Crossref
|
|
|
|
|
Sawadogo-Lingani H, Diawara B, Ganou L, Amua-Awa WK, Halm M, Jakobsen M (2003). Fermentation naturelle des graines de néré : aspects microbiologiques et modifications physico-chimiques. In Valorisation technologique et nutritionnelle du néré ou Parkia biglobosa (Jacq.) Benth: une espèce agro forestière, Diawara B. and Jakobsen M.(eds.), DANIDA-KVLCNRST/IRSAT pp. 107-116.
|
|
|
|
|
Terlabie NN, Sakyi-Dawson E, Amoa-Awua WK (2006). The comparative ability of four isolates of Bacillus subtilis to ferment soybeans into dawadawa. International Journal of Food Microbiology 106(2):145-152.
Crossref
|
|