Full Length Research Paper
ABSTRACT
In this study, the antioxidant activity and phytochemical analysis of extracts from the bark of Fagara zanthoxyloides and seeds of Mucuna pruriens were performed. Phytochemical analysis quantified total polyphenols, flavonoids, tannins and anthocyanins and the antioxidant activity was evaluated by DPPH method. The high levels of polyphenols are obtained from the aqueous extract of F. zanthoxyloïdes (488.6 ± 1.1 GAE/g) and the methanolic extract of Mucuna pruriens (142.9±12.9 GAE/g). The acetone extracts of F. zanthoxyloïdes and ethyl acetate extract of M. pruriens showed better flavonoid levels with 9.80±0.72 and 15.6±0.02 QE/g, respectively. For tannins, the aqueous extracts of F. zanthoxyloides and methanolic from M. pruriens showed a level of 19.2±1.41 and 5.7±0.74 CE/g. The antioxidant activity of the different extracts of the plants proved the highest antioxidant activity for the acetone extract of F. zanthoxyloïdes (IC50=3.44 mg/L). The methanolic extract of M. pruriens showed weak antioxidant activity IC50=3176.22 mg/L, the highest antioxidant activity of F. zanthoxyloïdes.
Key words: Mucuna pruriens, Fagara zanthoxyloïdes, polyphenols, antioxidant, in vitro activity, DPPH.
INTRODUCTION
Traditional medicine remains the main remedy for a large majority of African populations to solve their health problem, as it constitutes an important part of the cultural heritage. According to the World Health Organization, nearly 80% of populations depend on traditional medicine for primary health care (WHO, 2002). Medicinal plants are plants used in traditional medicine of which at least a part has medicinal properties, their actions come from their chemical compounds (primary or secondary metabolites) or from the synergy between the different compounds present. The universal role of plants in the treatment of disease is illustrated by their use in all major systems of medicine. Oxidative stress is involved in a wide spectrum of diseases which have a huge impact on the health of populations. These diseases causing oxidative stress are generally due to the excessive production of reactive oxygen species (ROS) and reactive nitrogen species (ERN) which could become toxic to major components of the cell: lipids, proteins, and nucleic acids; this would cause cellular dysfunction and would be involved in various pathologies such as: cardiovascular diseases, cancer, diabetes, neuro-degenerative diseases, and the aging process. A large number of scientific studies have been carried out to discover the functional properties of compounds of plant origin, antioxidant and others, which could be effective for health.
Fagara zanthoxyloïdes Lam (Rutaceae) is a plant that grows spontaneously in tropical Africa and preferably on cool, moist soils. Their roots are used and sold in African markets; they have a very popular spicy flavor and are used to calm dental pain. Its use as a toothpick is particularly recommended. This plant, well known to traditional therapists, is traditionally used to treat sickle cell crises by preventing hemolysis of red blood cells (Sofowara et al., 1979).
Mucuna pruriens (Linn.) belongs to Fabaceae family, commonly known as common cowith, cowhage, kavach, velvet bean, kapikachhu and naikaranam. It is an indigenous leguminous plant, is well known for producing itch. It is one of the most popular drugs in the Ayurvedic system of medicine. All parts of M. pruriens have valuable medicinal properties (Caius, 1989). The roots are bitter, sweet thermogenic, emollient, stimulant, purgative and diuretic. The seeds are astringent, laxative, antihelmentic, alexipharmic and tonic (Taylor, 2005). The leaves are broadly ovate, elliptic or rhomboid ovate, unequal at base. Leaves used as aphrodisiac, antihelmentic, tonic and are useful in stomach ulcers, inflammation, helminthiasis, cephalalgia and general debility (Sathyanarayanan and Arulmozhi, 2007). M. pruriens posses a wide range of pharmacological activities such as anti inflammatory (Hishikar et al., 1989), neuro-protective activity (Manyam et al., 2004), antioxidant activity (Tripathi and Upadhyay, 2001), antidiabetic (Davies, 1994; Dawan et al., 1980), anti protozoal activity (Rathi et al., 2002), and antimicrobial activity (Ekanem et al., 2004). Therefore, in the present investigation, efforts have been made to study the antioxidant activity of hexane, chloroform and methanolic extracts from M. pruriens leaves against various bacterial and fungal species in vitro.
The literature does not mention a phytochemical study and antioxidant activity of these two in the Central African Republic.
MATERIALS AND METHODS
Reagents and products
Products and reagents used for the various analyses were provided by Sigma-Aldrich-Fluka (Saint Quentin France).
Collection of plant material and preparation of extract
The plant materia l was collected in October 2020 in M’baïki(Boukoko) in the south of the Central African Republic. The specimen (PJK102020) was identified by botanist (Dr. Olivia Semboli) in the Department of Life Sciences and the Center for Studies in Pharmacopoeia and Traditional Medicine (CERPHAMETRA) from the University of Bangui. Extraction was done by macerating 200 g of sample in 2 L of each solvent for 4 h. The extracts were evaporated in a rotavapor at 35°C.
Determination of total phenolics
Total polyphenols were quantified using the method of Namkona et al. (2017), and then slightly modified. We mixed 20 μL of extract plus 100 μl of sodium carbonate (75 g/L in 20 ml H2O), the whole was mixed with 100 μl of 0.2 N Folin reagent. The whole was stirred for 30 min and then incubated for 15 min. We then read the absorbance at 765 nm. The blank was measured under the same conditions as the Folin reagent is replaced by water. Gallic acid (GA) was used as standard. The results are expressed as mg of gallic acid equivalent by gram of dry mass (dw).
Determination of flavonoids
The rate of flavonoids was obtained using the method described by Namkona et al. (2017). In 96-well microplates, 100 μL of different plant extracts were mixed with 100 μL of aluminum trichloride (AC13) in methanol (2%) and the absorbance was read at 415 nm after 15 min incubation at room temperature. The blank was measured under the same conditions by mixing 100 μL extract and 100 μL of methanol. Quercetin is used as a standard and is expressed as a quercetin equivalent by gram of dry mass.
Determination of tannins
The condensed tannins were quantified by mixing 50 μL with 150 μL of vanillin (1% H2SO4 in 7 M), according to the method of Namkona et al. (2017) and then slightly modified. The whole was incubated at 25°C for 15 min. The absorbance of the solution was measured at 500 nm. The measurement was made of for a blank when the vanillin was replaced with water. Catechin was used as standard. The results were expressed as mg of catechin equivalent by gram of dry mass.
Determination of total anthocyanins
Total anthocyanins were quantified in this study using the Namkona et al. (2017) pH difference method and then lightly modified. 20 μL of each extract was added to 180 μL of the pH 1.0 and 4.5 solutions of hydrochloric acid, potassium chloride (pH 1.0, 0.2 M) and acetic acid, sodium acetate. The corresponding absorbance was calculated using the following formula:
A = [(A510-A700) pH1.0- (A510-A700). pH4.5].
Molar extinction coefficient of 29600 was used. The results were expressed as mg of cyanidin-3-glucoside equivalent of dry mass.
Antioxidant activity by DPPH
The anti-radical activity of the extracts was determined by the method of Bekir et al. (2013) and then slightly modified. In a 96-well microplate, 20 μL of each extract and 180 μL of the DPPH solution were introduced. The mixture was stirred for 30 s and then incubated for 30 min in the dark. The reading was made at 524 nm.

The antioxidant activity of the extract is expressed as IC50 which defines the concentration of the extract that reduces by 50% the free radical (DPPH). Ascorbic acid was used as standard.
Data analysis
Data were processed by the software Excel and slopes, variances, standard deviations, and IC50 were determined. The threshold of 5% margin of error was used as a criterion for significance in all cases.
RESULTS AND DISCUSSION
Chemical composition of extracts
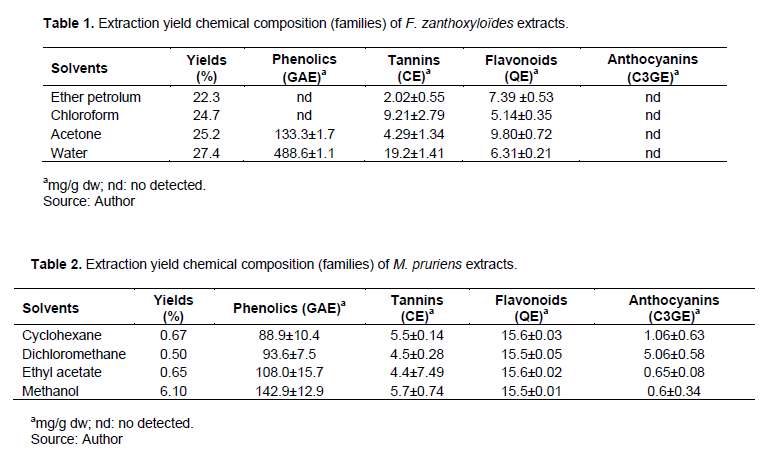
Table 1 shows the results of the phytochemical screening of F. zanthoxyloides. The best extraction yield is obtained with water and the low yield with petroleum ether with 27.4 and 22.3%, respectively. Bossokpi (2003) resulted in extraction yields of 4% (petroleum ether), 6% (dichloromethane) and 8% (methanol) and 1.8 (water) with the bark of the roots. Our yields are better compared to that of the latter results. This difference could be due to the organs used, since we worked on the bark of the trunk, on the other hand they used the bark of the roots. Four chemical families (polyphenols, flavonoids, tannins and anthocyanins) were quantified in this study. For petroleum ether extract, the polyphenols, flavonoids and tannins levels are in the order of 488.6 ± 1.1; 39 ± 0.53 and 2.028 mg/g ± 0.554, respectively. The chloroform extract gave the following results, 5.14 mg/g ± 0.35 for flavonoids and, 211 mg/g ± 2.795 for condensed tannins. The acetone extract contains 133.3 mg/g ± 13.7 polyphenols, 9.80 mg/g ± 0.72 flavonoids and 4.290 mg/g ± 1.347 condensed tannins. Finally, the aqueous extract quantified 6.31 mg/g ± 0.21 of flavonoids and 19.2 mg/g ± 1.412 of tannins. Adefisoye et al. (2012) demonstrated the presence of flavonoids and tannins in the roots of this plant in Nigeria. This confirms the flavonoids and tannins quantified in this study.
Table 2 shows the results of the phytochemical study of extracts from M. pruriens. For total polyphenols, the results are respectively 88.9 mg/g ± 10.4 (cyclohexane extract), 93.6 mg/g ± 7.5 (dichloromethane extract), 108.0 mg/g ± 15.7 (ethyl acetate extract) and 142.9 mg/g ± 12.9 for the methanol extract.
The methanolic extract contains more tannins than the other extracts with 5.7 mg/g ± 0.74. On the other hand, the highest rate of flavonoids is obtained with the acetone extract 9.80 mg/g ± 0.72 (Figure 4). Muhali et al. (2019) made it possible to find 3130.1 mg/g ± 15.5 for polyphenols, 63.3 mg/g ± 1.9 for flavonoids in aqueous and ethanolic extracts of mature fruits.
The levels of polyphenols and flavonoids of the latter are higher than those obtained in the present study. These differences could be due to the difference in part of the plant used. Also, the difference between the solvents used would affect the values of the quantified chemical compounds.
Antioxidant activity by DPPH
The histogram (Figure 1) shows the results of the antioxidant activity of the extracts and of the quercetin used as a reference molecule (Figure 3). The acetone extract showed strong antioxidant activity with IC50 equal to 3.44 mg/L, chloroform and aqueous extracts have IC50 values of 10.13 and 11.06 mg/L, respectively. The petroleum ether extract gave an IC50> 250 mg/L.
The IC50 value of acetone extract proves that this plant has a very high antioxidant activity than the reference molecule which has an IC50 of 6.3. Antioxidant activity was demonstrated by the work of Bossokpi (2012). Imaga et al. (2011) found an IC50 of 500 mg/L with the aqueous extract of the stems of F. zanthoxyloïdes. The difference between our results and that of the latter could be in the different parts of the plant used. Climatic and soil variations can influence chemical composition.
Figure 2 shows the IC50 values for the antioxidant activity of M. pruriens extracts. The best antioxidant activity was obtained with the methanol extract with 176.22 mg/L followed by the ethyl acetate extract 246.22 mg/L. The cyclohexane extract exhibited a lower IC50 with 1078.46 mg/L. The work of Ruhi et al. (2020) proved the antioxidant activity of M. pruriens. Kumar et al. (2010) demonstrated that extracts of M. pruriens were effective against free radicals. The antioxidant activity proven by the study is in line with this previous work.


CONCLUSION
This study allowed us to assess the antioxidant activity and quantify some chemical families (total polyphenols, tannins, flavonoids and anthocyanins) of F. zanthoxyloïdes and M. pruriens, used by different populations to treat themselves against various diseases.
It emerges from this study that these plants are rich in phenolic compounds which are very important in the fight against cardiovascular diseases. The antioxidant activity of extracts from these plants proven in this study is in accordance with their total polyphenol compositions. These plants could be used in the treatment of diseases linked to oxidative stress which presently poses a serious public health problem.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Bekir J, Mars M, Souchard JP, Bouajila J (2013). Assessment of anti-oxidant, anti-inflammatory, anti-cholinesterase and cytotoxic activities of pomegranate (Punica granatum) leaves. Food and Chemical Toxicology 55:470-475. |
|
|
Caius JF (1989). The Medicinal and poisonous legumes of India. Scientific Publishers, Jodhpur pp. 70-71. |
|
|
Davies J (1994). In activation of antibiotics and the dissemination of resistance genes. Science 264(5157):375-382. |
|
|
Dawan BN, Dubey MO, Gesa AA (1980). Screening of Indian plants for biological activity. Indian Journal of Experimental Biology 18:594-606. |
|
|
Ekanem Ap, Objekezie A, Klos W, Knoj K (2004). Effects of crude extracts of M. pruriens (Fabaceae) and Carica papaya (Caricaceae) against the protozoan fish parasite Icchthyophthiris multifiliis. Parasitology Research 92(5):361-366. |
|
|
Hishikar R, Shastry S, Shinde S, Guptha SS (1989). Preliminary photochemical and anti-inflammatory activity of seeds of M. pruriens. Indian Journal of Pharmacology 13(1):97-98. |
|
|
Kumar DS, Muthu AK, Smith AA, Manavalan R (2010). Free radical scavenging activity of various extracts of whole plant of Mucuna pruriens (Linn): an in vitro evaluation. Journal of Pharmacy Research 3(4):718-721. |
|
|
Manyam BV, Dhanasejkaran M, Hare TA (2004). Neuroprotective effects of the antiparkinson drug Mucuna pruriens. Phytotherapy Research 18(9):706-712. |
|
|
Muhali JO, Anthony JA, Francis BL (2019). "Antioxidant and phytochemical activities of Amaranthus caudatus L. harvested from different soils at various growth stages." Scientific Reports 9(1):1-14. |
|
|
Namkona AF, Bolevane Ouantinam SF, Moustapha F, Worowounga X, Ngaissona P, Koane JN, Syssa-Magalé JL (2017). Biological activities and phytochemical analysis of extracts Afrostyrax lepidophyllus Mildbr. seeds. Journal of Phytopharmacology 6(2):102-106. |
|
|
Rathi SS, Grover JK, Vats V (2002). The effects of Momardica charantia and M. pruriens in experimental diabetes and their effect on key metabolic enzymes involved in carbohydrate metabolism. Phytotherapy Research 16(8):774-777. |
|
|
Ruhi P, Prince C, Huma K, Ravinder K, Mohammed AK (2020). An assessment of potential nutritive and medicinal properties of Mucuna pruriens: a natural food legume. 3 Biotech 10(261):2-15. |
|
|
Sathyanarayanan L, Arulmozhi S (2007). Mucuna pruriens Linn. A Comprehensive Review. Pharmacognosy Reviews 1(1):157-162. |
|
|
Sofowora EA, Isaac-Sodeye WA, Ogunkoya LO (1979). Isolation and characterization of an antisickling agent from the root of Fagara zanthoxyloides. In Proceedings of a Symposium Fagara and the Red Blood Cell (pp. 79-87). University of Ife Press. |
|
|
Taylor L (2005). The Healing power of Rainforest Herbs 444 p. |
|
|
Tripathi YB, Upadhyay AK (2001). Antioxident property of Mucuna pruriens Linn. Current Science 80(11):1377-1378. |
|
|
World Health Organization (2002). Programme on Traditional Medicine. Stratégie de l'OMS pour la médecine traditionnelle. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0