ABSTRACT
Rubber seeds from a Nigerian hybrid rubber tree were dried in an oven at 110°C for 2 h to 7 wt% moisture content and cold-screw pressed at 30°C to obtain an oil yield of 28 wt%. Chemical compositional, nuclear magnetic resonance (NMR) and Fourier transform infra-red spectroscopy (FTIR) analyses were conducted on the oil sample extracts. Chemical analysis of the oil indicated iodine value of 136.07 g I2/100 g oil, peroxide value of 9.45 O2/kg oil, saponification value of 189.5 mg KOH/g oil, and confirms its suitability for applications, such as biodiesel production, oleochemicals synthesis, polyurethane composites and water-reducible alkyd resins, pharmaceutical products, plasticizers, adhesives, and surfactants. Rubber seed oil is a promising substitute to linseed oil as semi-drying oil for oily paint formulation. 1H NMR analysis revealed that the fatty acid compositions consist of linoleic acid (34.22 wt%), oleic acid (28.6 wt%), linolenic acid (18.6 wt%), and saturated fatty acids (18.57 wt%). FTIR analysis indicated fingerprint regions of 1461 to 585 cm-1 which can be used to check adulteration of the oil. The NMR spectra (1H and 13C) of the oil are similar to those of other vegetable oils with well-identified peaks and regions that can be used to authenticate the quality of the oil.
Key words: Rubber seed oil, characterization, nuclear magnetic resonance (NMR), Fourier transform infra-red spectroscopy (FTIR), quality assessment, technical applications.
Edible vegetable oils have several food applications and they are increasingly being used for other purposes such as resinous pigments and drying oils in paints and coatings production (Aigbodion and Bakare, 2005). To reduce costs associated with the use of edible vegetable oils in the food industry, non-edible oils of good industrial applications are highly sought to replace edible oils (Roschat et al., 2017). In recent times, with the development of new technologies and research future areas expansion on non-edible oils applications to replace edible oils, detailed characteristic property assessment of oils quality are imperative to the entrepreneurs (Reshad et al., 2015). Therefore, novel oils characterization to determine their possible applications and to forestall adulteration because of price differentials with low quality oils is paramount for high quality derivable products (Barison et al., 2010).
Recently, several researchers studied rubber seed oil (RSO) usefulness to authenticate its industrial application as a renewable resource material to complement the fast depleting non-renewable mineral oils of fossil origins (Onoji et al., 2019). Rubber tree accounts for 99% of world’s natural rubber (NR) latex used for the production of several rubber products (Atabani et al., 2013). Currently, the seeds are underutilized and allowed to rot away in the plantations, apart from a minimal use in subsequent tree plant breeding process in Nigeria (Onoji et al., 2017). The rubber seeds used in this study were collected from the 40-hectare (ha) NIG800 series plantations of Rubber Research Institute of Nigeria (RRIN), Iyanomo, Benin-City (Aigbodion and Bakare, 2005). The foreign (Malaysia, Sri Lanka, etc.) rubber seeds (RRIM 600, RRIM 501, PB 28/59, PB 5/63, RRIM 628, RRIC 45, RRIM 614, AVROS 1581, RRIM 605, PB 5/51, GT1, RRIM 62, RRIM707, PR107 and PB217) used as parentage stock in these plantations were pre-germinated in pre-nursery beds (Umar et al., 2010). Ground and polythene bags nursery techniques were used for seedlings production. Certified rubber seedlings obtained through budding techniques were used for planting in these plantations. Specie of Nigerian hybrid is a high-yield latex (3000 to 3500 dry NR/hectare/year) tree, resistant to wind and with a capacity to produce about 1200 seeds/tree/year (Onoji et al., 2020). The specie has an average weight of 4 g/seed (Onoji et al., 2019), and a non-edible oil content yield of about 43 wt% (Onoji et al., 2016). Non-edible seed oil from rubber tree has been identified as a potentially promising material for several industrial applications such as, the production of biodiesel (Yang et al., 2011), oleochemicals (Hosamani and Katagi, 2008), polyurethane composites and water-reducible alkyd resins (Bakare et al., 2010), pharmaceutical products, plasticizers, adhesives, and surfactants (Onoji et al., 2016). De-oiled cake, after pretreatment to remove toxic material such as hydrogen cyanide, is suitable as a valuable source of proteins for farm animals and poultry feeds (Eka et al., 2010). RSO has been characterized as a semi-drying oil (Aravind et al., 2015), and of a high quality that makes it a promising substitute for linseed oil in paint formulation (Ebewele et al., 2010). In Nigeria, linseed oil is imported for use in the paint industry and other applications (Okiemen et al., 2005). However, available report shows that RSO was yet to have any commercial value in Nigeria, which has the capacity to produce 13,000 tons RSO/year (Okiemen et al., 2005). The oil can be processed into a semi-drying oil for the paint industry to reduce importation of linseed oil which depletes the Nigerian foreign exchange reserves. There is therefore a need to characterize the Nigerian hybrid RSO in order to authenticate its quality against any adulteration with respect to the aforementioned usage.
Vegetable oils authentication for product quality via spectroscopic methods use is well reported in literature (Sadowska et al., 2008). Gas chromatography coupled with mass spectrometry (GC-MS) has been in use for several decades (Skooge et al., 2007). However GC-MS, a destructive method, involves oil sample chemical modification (oxidation) with the tendency to produce unreliable results. In addition, GC-MS is cumbersome, cost in-effective, time consuming and may pose problems in result interpretations, especially the fatty acid compositions (Barison et al., 2010). Fourier transform infra-red spectroscopy (FTIR) is a non-destructive technique reported for use in the analysis of free fatty acids and to monitor oil quality to check adulteration (Valente et al., 2016) through the identification of the functional groups present in the sample (Bohre, 2013). Proton (1H) and carbon-13 (13C) nuclear magnetic resonance (NMR) spectroscopy is a non-destructive technique used to determine the proportion of different acyl groups present in oils and fats, and other liquids in a very short time for sample preparation and experimental spectrum acquisition compared to GC-MS (Scano et al., 2008; Guillén and Ruiz, 2003a). 1H NMR spectra area signals are proportional to the number of hydrogen atoms that produce the signals (Guillén and Ruiz, 2003a). This method has many advantages because it does not involve chemical modification of sample like the GC-MS (Yeung et al., 2008).
In this study, seed oil was extracted by mechanical means notable for high-quality oil (Ebewele et al., 2010). This method is cost effective and can be adapted to small- to medium-scale entrepreneurship due to its low operating and maintenance costs. For instance, a demonstration-scale rubber seed oil mechanical extractor designed and fabricated by RRIN located in Benin City at the cost of US$1,050 has a capacity to extract 500 L of oil/day. The extracted oil physico-chemical properties were determined by using standard methods described elsewhere (Onoji et al., 2016), while FTIR and NMR spectroscopy were deployed to determine oil functional groups and fatty acids, respectively.
Materials and reagents
Fresh and glossy rubber seeds used for this study were handpicked from the hybrid rubber estate plantation of RRIN. All reagents from BDH Chemicals Ltd., Poole England, and GFS Chemicals, Inc., 867 McKinley Ave., Columbus, OH 43223 and used in the analyses were of analytical grades.
Seed oil extraction
Seed kernels were separated from seed shells by gentle cracking using a laboratory mortar and pestle. The kernels were weighed, and dried in an oven at 110°C for 2 h to attain constant weight for moisture content determination. About 2 kg of fresh seed kernel was dried at 110°C to 7 wt% moisture content for a maximum oil yield (Igeleke and Omorusi, 2007). Seed oil was extracted at 30°C from the dried kernel using a mechanical oilseed screw press. Extracted oil was stored in screwed airtight plastic containers for physico-chemical characterization, and spectroscopic analysis.
Physico-chemical analysis of rubber seed oil
The standard methods adopted for the determination of physico-chemical properties of the rubber seed oil extracted using a 99.9% n-hexane solvent in a soxhlet extractor were obtained elsewhere (Onoji et al., 2016). The experiments were carried out in duplicate, and the average values recorded for accuracy of data.
Spectroscopic analysis of rubber seed oil
Oil infrared spectra were recorded using an FTIR spectrometer (Model: TENSOR 27, Bruker Optics Inc., USA) equipped with a detector, and interfaced to a personal computer operating under OPUS spectroscopy software supplied together with the instrument. A film of 2 μL of the RSO sample was deposited between two disks of KBr, in the absence of air (Guillén and Cabo, 2002). Sinclair et al. (1952) reported that the degree of unsaturation in vegetable oils correlates with some absorbance bands in the FTIR spectrum, hence the frequencies of such bands are closely related to the proportions of saturated, monounsaturated, and polyunsaturated acyl groups (Guillén and Ruiz, 2003a). Consequently all spectra were recorded between 4000 and 500 cm-1.
Extracted oil 1H NMR spectrum was recorded on Bruker ultra-shield TM 500-MHz NMR spectrophotometer. Approximately 0.2 g of oil sample was dissolved in 500 μL of deuterated chloroform (CDCl3) as solvent (δ = 7.26 ppm) containing a small amount of tetramethylsilane (TMS) as an internal standard (δ = 0 ppm). This was then placed in 5 mm diameter NMR test tubes to commence the analysis (Guillén and Ruiz, 2003a). The acquisition data were: spectral width 8.1 kHz, relaxation delay 3s, 15 scans, and pulse width 30°.
13C NMR experiments were also carried out with Bruker ultra-shield TM 500-MHz NMR equipment. About 25 mg of RSO was dissolved in 1 mL of CDCl3. Samples were placed in 5 mm NMR tubes at 20°C and analyzed within 2 days as described by Scano et al. (2008). The experiments were conducted at room temperature (28°C). 13C spectra were recorded at 235.2 ppm spectral width, relaxation delays 3s, and a total of 350 scans. The carbon atom in CDCl3 was observed at 77.42 ppm. All other peaks were assigned with respect to it.
Fatty acid compositions of rubber seed oil using NMR spectroscopy
Unsaturation degree in oils and fats can also be determined from the proportion of olefinic hydrogen atoms obtained from 1H NMR data, besides different acyl groups present in oil sample (Guillén and Ruiz, 2003b). The proportions of Linolenic (Ln), Linoleic (L), Oleic (O), and saturated (S) fatty acids present in the RSO were calculated from the areas of the identified peaks (1 – 10) in 1H NMR spectra (Figure 1) using parameters shown in Equations 1 to 4 for oils containing similar acyl groups as that mentioned previously (Reshad et al., 2015; Guillén and Ruiz, 2003b).
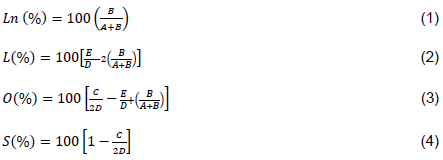
where A, is the area signal 1, corresponding to methyl hydrogen atoms of saturated, oleic (omega-9), and linoleic acyl groups; B is area signal 2, corresponding to methyl hydrogen atoms of linolenic acyl groups; C is area signal 5, corresponding to methylenic hydrogen atoms in position α, in relation to one double bond (allylic protons); D is area signal 6, corresponding to methylenic hydrogen atoms in position α, in relation to the carboxyl group; and E is area signal 7, corresponding to methylenic hydrogen atoms in α position with two double bonds (bis-allylic protons).
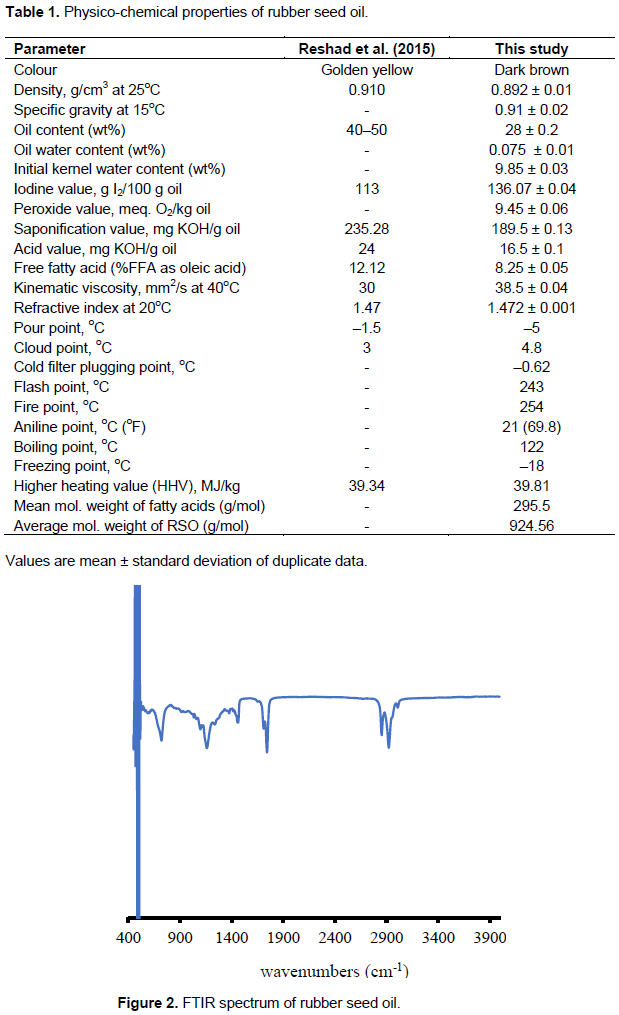
Physico-chemical properties of rubber seed oil
Mechanically extracted hybrid oil properties are presented in Table 1, with oil yield of 28 wt%. This yield closely agrees with the yield of 23 wt% reported by Aigbodion and Bakare (2005) on similar experiment with RSO. The oil parameters such as iodine value of 136.07 g I2/100 g oil, peroxide value of 9.45 O2/kg oil, saponification value of 189.5 mg KOH/g oil, and refractive index of 1.472 are within the range reported by other researchers on RSO (Reshad et al., 2015; Aravind et al., 2015).
FTIR and NMR spectroscopic analysis of rubber seed oil
Results obtained from oil FTIR and NMR analysis are geared towards providing an insight into the fatty acid structural configuration and, compositions that enables easy evaluation of its potential applications by entrepreneurs.
FTIR analysis of rubber seed oil
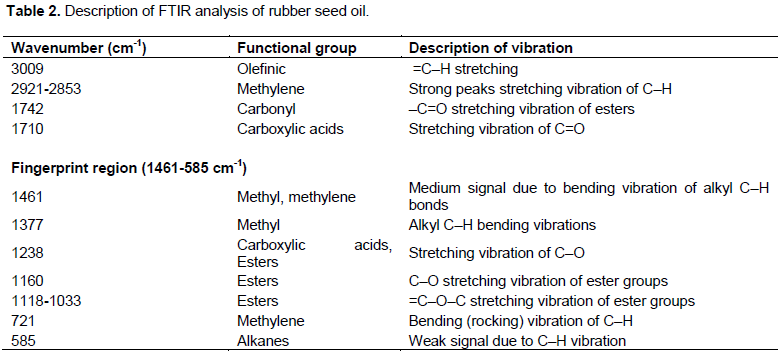
Figure 2 shows the FTIR spectrum of the extracted oil. The main peaks and their assignment to functional groups are presented in Table 2. The O-H stretching vibrations bands (for alcohols, carboxylic acids, and hydroperoxides) usually in the absorption range of 3200 to 3600 cm-1 for vegetable oils (Ogbu and Ajiwe, 2016) are absent in the RSO used for this study. The observed spectrum implies that triglyceride is the main component of the oil as a result of the strong presence of ester carbonyl group (C=O) at 1742 cm-1. Ester group presence could be attributed to the stretching vibrations of C–O observed at 1160 cm-1. Results on fingerprint obtained are within the range (1461 to 585 cm-1) which provides useful information to detect oil adulteration. The results obtained are within the range reported by other researchers on RSO (Reshad et al., 2015).
NMR spectra analysis of rubber seed oil
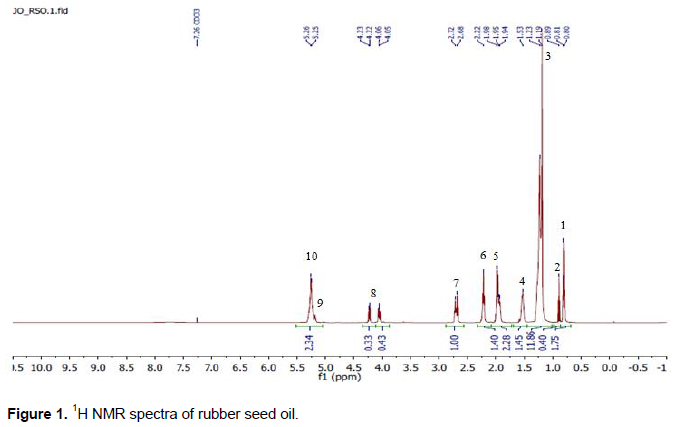
Figure 1 depicts the 1H NMR spectrum of RSO. The assignment of signals is listed in Table 3. Signal 1 is the overlap of doublet signals of methyl group protons that appears between 0.8 and 0.81 ppm. Signal 2 is a singlet methyl group proton, and appears at 0.89 ppm. Signal 3 appears between 1.19 and 1.23 ppm corresponding to linolenyl chains protons, and saturated methylene group of acyl chains, respectively. Signal 4 appears at 1.53 ppm, representing the methylene protons in the β position. Signal 5 appears between 1.94 and 1.98 ppm, due to α-methylene proton related to a protons in α position in relation to a single double bond (allylic protons). Signal 6 occurred due to the methylene protons in α position, and it appears at 2.22 ppm. Signal 7 is a signal overlap between 2.68 and 2.72 ppm, due to responses from α-methylene protons related to the double bonds (bi-allylic protons). Signal 8 appears at 4.05, 4.06 and 4.22, 4.23 ppm, due to the protons on carbon atoms 1 and 3 of the glyceryl group. Signal 9 appears at 5.25 ppm, corresponding to the proton on carbon 2 atom of the glyceryl group, and it overlaps with Signal 10 at 5.26 ppm that represents olefinic protons of different acyl groups.
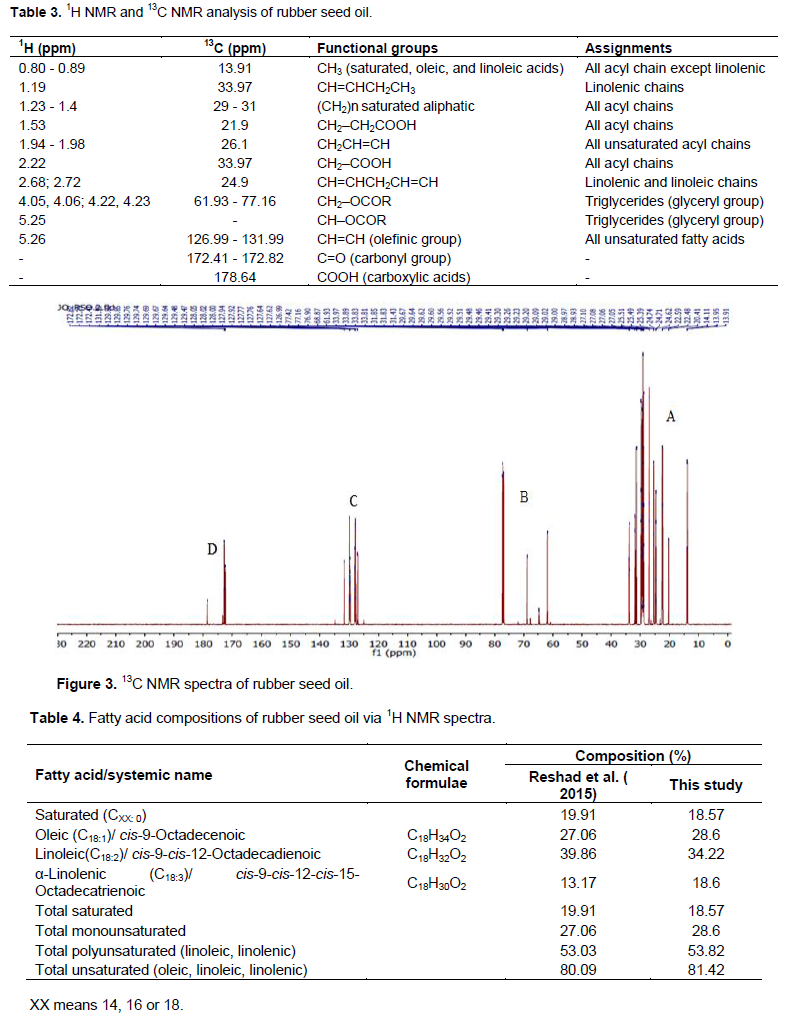
13C NMR spectra analysis of rubber seed oil
Figure 3 shows the 13C NMR spectrum of the oil under analysis with well-defined four distinct regions (A, B, C and D): A (13.91 - 33.97 ppm) due to methyl or alkyl functional groups for saturated acids, oleic, and linoleic acids; B (61.93 - 77.16 ppm) due to glyceryl C-atoms; C (126.99 - 131.99) due to the presence of unsaturated alkenes; and D (172.41 - 178.64) due to the presence of carbonyl and carboxylic acid groups. The assignment of signals is listed along with those from 1H NMR spectrum in Table 3. Both 1H and 13C NMR spectra of the oil are similar to those of known vegetable oils (Sadowska et al., 2008; Guillén and Ruiz, 2003b).
Fatty acid compositions of rubber seed oil through 1H NMR spectra
The fatty acid compositions of vegetable oils depend to a large extent on the seed storage and processing time, extraction technique employed, and method of oil analysis etc. Based on Equations 1 to 4 and the identified peak areas of the 1H NMR spectrum (Figure 1), the fatty acids composition was quantified and tabulated in Table 4. The spectra analysis showed that the fatty acid compositions of the extracted RSO consist of linoleic acid (34.22 wt%), oleic acid (28.6 wt%), linolenic acid (18.6 wt%), and saturated fatty acids (18.57 wt%), and it compared favorably with results on RSO from other researchers (Reshad et al., 2015). The results show that linoleic acid is the predominant fatty acid present in this hybrid RSO and over 80% of the fatty acids are unsaturated.
Conversely, the oil may be categorized among the less nutritional quality due to the low profile of oleic acid (28.6 wt%) compared to olive oil (64.6 - 84.4 wt%) and canola (60 - 75 wt%) (Reshad et al., 2015). In addition, due to the high content of unsaturated fatty acids coupled with the low temperature properties, the oil is a promising material for biodiesel production, and as a substitute for linseed oil suitable for making drying oil for paint and varnish formulations.
In this study, rubber seed oil was mechanically extracted from the seeds of Nigerian hybrid rubber trees with oil yield of 28 wt%. An Adulteration of high-value RSO with low-grade oils often constitutes economic and commercial loss. Consequently chemical analysis to check for adulteration revealed that the RSO is non-edible and possesses significant potentials as substitute to the edible oils on different industrial applications. For instance, the oil can be used as a replacement for linseed oil as a semi-drying oil in the paint industry. The spectroscopic analytical methods (FTIR and NMR spectroscopy) employed in the study are non-destructive, simple, fast, reliable, cost-effective, and with no sample pre-treatment required compared to the GC-MS analytical tool which involves chemical modification of oil samples. The 1H NMR spectra analysis of the oil shows high degree of unsaturation which implies susceptibility to low temperature, hence suitable for low climatic regions. The observed FTIR fingerprint regions of the oil are adequate for adulteration detection of low quality RSO that may impair derived products. The –C=O, =C–O–C, and C–O vibrations in the FTIR bands of 1742, 1118-1033, and 1160 cm-1 indicate the presence of strong ester groups supportive of biodiesel production. The findings of this study are useful parametric data for rubber seed oil identification, quantification, and authentication purposes.
The authors have not declared any conflict of interests.
The authors appreciate the support provided by the Petroleum Training Institute, Effurun, Nigeria, and the University of the Witwatersrand (Wits), Johannesburg, South Africa.
REFERENCES
|
Aigbodion AI, Bakare IO (2005). Rubber seed oil quality assessment and authentication. Journal of the American Oil Chemists' Society 82(7):465-469.
Crossref
|
|
|
|
Aravind A, Joy ML, Nair KP (2015). Lubricant properties of biodegradable rubber tree seed (Hevea brasiliensis Muell. Arg) oil. Industrial Crops and Products 74:14-19.
Crossref
|
|
|
|
|
Atabani AE, Silitonga AS, Ong HC, Mahlia TMI, Masjuki HH, Badruddin IA, Fayaz H (2013). Non-edible vegetable oils: A critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renewable and Sustainable Energy Reviews18:211-245.
Crossref
|
|
|
|
|
Bakare IO, Okieimen FE, Pavithran C, Abdul Khalil HPS, Brahmakumar M (2010). Mechanical ant thermal properties of sisal fiber-reinforced rubber seed oil-based polyurethane composites. Material Design 31:4274-4280.
Crossref
|
|
|
|
|
Barison A, Pereira da Silva CW, Campos FR, Simonelli F, Lenz CA, Ferreira AG (2010). A simple methodology for the determination of fatty acid composition in edible oils through 1H NMR spectroscopy. Magnetic Resonance in Chemistry 48:642-650.
Crossref
|
|
|
|
|
Bohre A (2013). Immobolization of radioactive waste in ceramic-based hosts: Radioactive waste immobolization. Hamburg: Anchor Academic Publishers.
|
|
|
|
|
Ebewele RO, Iyayi AF, Hymore FK (2010). Considerations of the extraction process and potential technical applications of Nigerian rubber seed oil. International Journal of Physical Sciences 5(6):826-831.
|
|
|
|
|
Eka HD, Tajul Aris Y, Wan Nadiah WA (2010). Potential use of Malaysia rubber (Hevea brasiliensis) seed as food, feed and biofuel. International Food Research Journal 17:527-534.
|
|
|
|
|
Guillén MD, Cabo N (2002). Fourier transform infrared spectra data versus peroxide and anisidine values to determine oxidative stability of edible oils. Food Chemistry 77:503-510.
Crossref
|
|
|
|
|
Guillén MD, Ruiz A (2003a). 1H nuclear magnetic resonance as a fast tool for determining the composition of acyl chains in acylglycerol mixtures. European Journal of Lipid Science and Technology 105:502-507.
Crossref
|
|
|
|
|
Guillén MD, Ruiz A (2003b). Rapid simultaneous determination by proton NMR of unsaturation and composition of acyl groups in vegetable oils. European Journal of Lipid Science and Technology 105:688-696.
Crossref
|
|
|
|
|
Hosamani KM, Katagi KS (2008). Characterization and structure elucidation of 12-hydroxyoctadec-cis-9-enoic acid in Jatropha gossypifolia and Hevea brasiliensis seed oils: a rich source of hydroxy fatty acid. Chemistry and Physics of Lipids 152:9-12.
Crossref
|
|
|
|
|
Igeleke CL, Omorusi VI (2007). Review of post-harvest deterioration of rubber Seeds. Journal of Agriculture and Social Research 7(2):11-19.
Crossref
|
|
|
|
|
Ogbu IM, Ajiwe VIE (2016). FTIR studies of thermal stability of the oils and methyl esters from Afzelia africana and Hura crepitans seeds. Renewable Energy 96:203-208.
Crossref
|
|
|
|
|
Okiemen FE, Pavithran C, Bakare IO (2005). Epoxidation and hydroxlation of rubber seed oil: one-pot multi-step reactions. European Journal of Lipid Science and Technology 107:330-336.
Crossref
|
|
|
|
|
Onoji SE, Iyuke SE, Igbafe AI (2016). Hevea brasiliensis (Rubber seed) oil: Extraction, characterization, and kinetics of thermo-oxidative degradation using classical chemical methods. Energy Fuels 30(12):10555-10567.
Crossref
|
|
|
|
|
Onoji SE, Iyuke SE, Igbafe AI, Daramola MO (2017). Transesterification of rubber seed oil to biodiesel over a calcined waste rubber seed shell catalyst: Modeling and optimization of process variables. Energy Fuels 31(6):6109-6119.
Crossref
|
|
|
|
|
Onoji SE, Iyuke SE, Igbafe AI, Daramola MO (2019). Hevea brasiliensis (Rubber seed) oil: Modeling and optimization of extraction process parameters using response surface methodology and artificial neural network techniques. Biofuels 10(6):677-691.
Crossref
|
|
|
|
|
Onoji SE, Iyuke SE, Igbafe AI, Daramola MO (2020). Rubber seed (Hevea brasiliensis) oil biodiesel emission profiles and engine performance characteristics using a TD202 diesel test engine. Biofuels 1-8, doi: 10.1080/17597269.2020.1738679.
Crossref
|
|
|
|
|
Reshad AS, Tiwari P, Goud VV (2015). Extraction of oil from rubber seeds for biodiesel application: Optimization of parameters. Fuel 150:636-644.
Crossref
|
|
|
|
|
Roschat W, Siritanon T, Yoosuk B, Sudyoadsuk T, Promarak V (2017). Rubber seed oil as potential non-edible feedstock for biodiesel production using heterogeneous catalyst in Thailand. Renewable Energy 101:937-944.
Crossref
|
|
|
|
|
Sadowska J, Johansson B, Johannessen E, Friman R, Broniarz-Press L, Rosenholm JB (2008). Characterization of ozonated vegetable oils by spectroscopic and chromatographic methods. Chemistry and Physics of Lipids 151:85-91.
Crossref
|
|
|
|
|
Scano P, Rosa A, Marincola FC, Locci E, Melis MP, Dessi MA, Lai A (2008). 13C NMR, GC and HPLC characterization of lipid components of the salted and dried mullet (Mugil cephalus) roe "bottarga". Chemistry and Physics of Lipids 151:69-76.
Crossref
|
|
|
|
|
Sinclair RG, Mckay AF, Myers GS, Jones RN (1952). The infrared absorption spectra of unsaturated fatty acids and esters. Journal of the American Chemical Society 74:2578-2585.
Crossref
|
|
|
|
|
Skooge DA, Holler FJ, Crouch SR (2007). Principle of Instrumental Analysis (6th Ed.). Thomson Publishing, USA.
|
|
|
|
|
Umar HY, Esekhade TU, Idoko SO, Ugwa IK (2010). Production analysis of budded rubber stumps in Rubber Research Institute of Nigeria (RRIN). Journal of Agricultural Science 1(2):109-113.
Crossref
|
|
|
|
|
Valente VSB, dos S. Vieira A, Teixeira RM (2016). Physicochemical characterization of commercial biodiesel/diesel blends and evaluation of unconventional spectroscopic vibrational techniques in the monitoring of their oxidation and hydrolysis during storage. Energy Fuels 30:8399-8409.
Crossref
|
|
|
|
|
Yang R, Su M, Zhang J, Jin F, Zhu C, Li M, Hao X (2011). Biodiesel production from rubber seed oil using poly (sodium acrylate) supporting NaOH as a water-resistant catalyst. Bioresource Technology 102:2665-2671.
Crossref
|
|
|
|
|
Yeung DKW, Lam SL, Griffith JF, Chan ABW, Chen Z, Tsang PH, Leung PC (2008). Analysis of bone marrow fatty acid composition using high-resolution proton NMR spectroscopy. Chemistry and Physics of Lipids 151:103-109.
Crossref
|
|